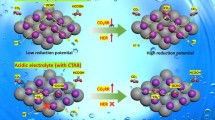
Abstract
Influence of chemical composition of the ionomers (polyvinyl alcohol (PVA) or Nafion®) on the oxygen reduction reaction (ORR) kinetics has been studied. The 5 wt% Nafion-Vulcan showed higher electrochemical activity toward ORR compared with that for the 5 wt% PVA-Vulcan. Four different Nafion® amounts were used to intermixing a carbide-derived carbon (CDC) or Pt-modified CDC catalysts and the highest electrochemical activity toward ORR was established for the 30 wt% Nafion-Pt/CDC catalyst. Influence of the different amounts of Nafion® ionomer in the catalyst is moderate compared to the effect of variation of the carbon support (Vulcan vs. CDC) or the ionomer (PVA vs. Nafion®). The Randles–Ševcik relationship was used to estimate the effective electrochemical active surface area (S eff) of the electrodes, depending on the chemical composition of the ionomer studied.












Similar content being viewed by others
References
Wilson MS, Valerio JA, Gottesfeld S (1995) Low platinum loading electrodes for polymer electrolyte fuel cells fabricated using thermoplastic ionomers. Electrochim Acta 40(3):355–363
Jung HY, Cho KY, Sung KA, Kim WK, Kurkuri M, Park JK (2007) Sulfonated poly (arylene ether sulfone) as an electrode binder for direct methanol fuel cell. Electrochim Acta 52:4916–4921
Holdcroft S (2014) Fuel cell catalyst layers: a polymer science perspective. Chem Mater 26:381–393
Antolini E, Giorgi L, Pozio A, Passalacqua E (1999) Influence of Nafion loading in the catayst layer of gas-diffusion electrodes for PEFC. J Power Sources 77:136–142
Viswanathan B, Helen M (2007) Is Nafion the only choice? Bulletin of the Catalysis Society of India 6:50–55
Litster S, McLean G (2004) PEM fuel cell electrodes. J Power Sources 130:61–76
Zhang X-Y, Ding Y-H (2014) Thickness-dependent structural and transport behaviors in the platinum–Nafion interface: a molecular dynamics investigation. RSC Adv 4:44214–44222
Sasikumar G, Ihm JW, Ryu H (2004) Dependence of optimum Nafion content in catalyst layer on platinum loading. J Power Sources 132:11–17
Passalacqua E, Lufrano F, Squadrito G, Patti A, Giorgi L (2001) Nafion content in the catalyst layer of polymer electrolyte fuel cells: effects on structure and performance. Electrochim Acta 46:799–805
Antoine O, Bultel Y, Durand R (2001) Oxygen reduction reaction kinetics and mechanism on platinum nanoparticles inside Nafion®. J Electroanal Chem 499:85–94
Park Y-C, Kakinuma K, Uchida H, Watanabe M, Uchida M (2015) Effects of short-side-chain perfluorosulfonic acid ionomers as binders on the performance of low Pt loading fuel cell cathodes. J Power Sources 275:384–391
Park Y-C, Tokiwa H, Kakinuma K, Watanabe M, Uchida M (2016) Effects of carbon supports on Pt distribution, ionomer coverage and cathode performance for polymer electrolyte fuel cells. J Power Sources 315:179–191
Siroma Z, Fujiwara N, Ioroi T, Yamazaki S, Yasuda K, Miyazaki Y (2004) Dissolution of Nafion membrane and recast Nafion film in mixtures of methanol and water. J Power Sources 126:41–45
Jung HY, Cho KY, Lee YM, Park JK, Choi JH, Sung YE (2007) Influence of annealing of membrane electrode assembly (MEA) on performance of direct methanol fuel cell (DMFC). J Power Sources 163:952–956
Zook LA, Leddy J (1996) Density and solubility of nafion: recast, annealed, and commercial films. Anal Chem 68:3793–3796
Marten ML (2004) Encyclopedia of polymer science and technology, in: Kroschwitz JI (ed). 3rd edn. Wiley, New York Volume 8:399–436
Ye Y-S, Rick J, Hwang B-J (2012) Water soluble polymers as proton exchange membranes for fuel cells. Polymers 4(2):913–963. doi:10.3390/polym4020913
Silva R, Muniz EC, Rubira AF (2008) Multiple hydrophilic polymer ultra-thin layers covalently anchored to polyethylene films. Polymer 49:4066–4075
Zugic DL, Perovic IM, Nikolic VM, SLJ M, Marceta Kaninski MP (2013) Enhanced performance of the solid alkaline fuel cell using PVA-KOH membrane. Int J Electrochem Sci 8:949–957
Masa J, Batchelor-McAuley C, Schuhmann W, Compton RG (2014) Koutecky-Levich analysis applied to nanoparticle modified rotating disk electrodes: electrocatalysis or misinterpretation? Nano Res 7(1):71–78
Ward KR, Gara M, Lawrence NS, Seth Hartshorne R, Compton RG (2013) Nanoparticle modified electrodes can show an apparent increase in electrode kinetics due solely to altered surface geometry: the effective electrochemical rate constant for non-flat and non-uniform electrode surfaces. J Electroanal Chem 695:1–9
Ward KR, Compton RG (2014) Quantifying the apparent ‘Catalyticʼ effect of porous electrode surfaces. J Electroanal Chem 724:43–47
Menshykau D, Streeter I, Compton RG (2008) Influence of electrode roughness on cyclic voltammetry. J Phys Chem C 112:14428–14438
Jänes A, Thomberg T, Kurig H, Lust E (2009) Nanoscale fine-tuning of porosity of carbide derived carbon prepared from molybdenum carbide. Carbon 47:23–29
Chai GS, Yoon SB, Yu J-S, Choi J-H, Sung Y-E (2004) Ordered porous carbons with tunable pore sizes as catalyst supports in direct methanol fuel cell. J Phys Chem B 108:7074–7079
Álvarez G, Alcaide F, Miguel O, Calvillo L, Lázaro MJ, Quintana JJ, Calderón JC, Pastor E, Esparbé I (2010) Technical electrodes catalyzed with PtRu on mesoporous ordered carbons for liquid direct methanol fuel cells. J Solid State Electrochem 14:1027–1034
Jäger R, Härk E, Steinberg V, Lust E (2016) Influence of temperature on the oxygen electroreduction activity at micro-mesoporous carbon support. J Electrochem Soc 163(3):F284–F290
Jäger R, Härk E, Kasatkin PE, Lust E (2014) Investigation of a carbon-supported Pt electrode for oxygen reduction reaction in 0.1 M KOH aqueous solution. J Electrochem Soc 161(9):F861–F867
Härk E, Jäger R, Lust E (2015) Effect of platinum nanoparticle loading on oxygen reduction at Pt Nanocluster activated microporous-mesoporous carbon support. Electrocatal 6:242–254
Härk E, Jäger R, Kasatkin PE, Steinberg V, Romann T, Möller P, Kanarbik R, Aruväli J, Kirsimäe K, Lust E (2015) Oxygen electrocatalysis on high-surface area non-Pt metal modified carbon catalysts. ECS Trans 64(36):11–21
Zhang J, Tang S, Liao L, Yu W, Li J, Seland F, Haarberg GM (2014) Improved catalytic activity of mixed platinum catalysts supported on various carbon nanomaterials. J Power Sources 267:706–713
Seah MP, Gilmore IS, Spencer SJ (2001) Quantitative XPS: I. Analysis of X-ray photoelectron intensities from elemental data in a digital photoelectron database. J Electron Spectrosc Relat Phenom 120:93–111
Fairley N, CasaXPSversion 2.3.12 www.casaxps.com
Datsyuk V, Kalyva M, Papagelis K, Parthenios J, Tasis D, Siokou A, Kallitsis I, Galiotis C (2008) Chemical oxidation of multiwalled carbon nanotubes. Carbon 46:833–840
Diaz J, Paolicelli G, Ferrer S, Comin F (1996) Separation of the sp3 and sp2 components in the C1s photoemission spectra of amorphous carbon films. Phys Rev B Condens Matter 54:8064–8069
Payne BP, Biesinger MC, McIntyre NS (2011) X-ray photoelectron spectroscopy studies of reactions on chromium metal and chromium oxide surfaces. J Electron Spectrosc Relat Phenom 184:29–37
Beverly S, Seal S, Hong S (2000) Identification of surface chemical functional groups correlated to failure of reverse osmosis polymeric membranes. J Vac Sci Technol 18:1107–1113
Chen C, Levitin G, Hess DW, Fuller TF (2007) XPS investigation of Nafion® membrane degradation. J Power Sources 169(2):288–295
Joo JB, Kim YJ, Kim W, Kim P, Yiet J (2008) Simple synthesis of graphitic porous carbon by hydrothermal method for use as a catalyst support in methanol electro-oxidation. Catal Commun 10(3):267–271
DeLuca NW, Elabd YA (2006) Nafion®/poly (vinyl alcohol) blends: effect of composition and annealing temperature on transport properties. J Membrane Science 282:217–224
Jiang L, Hsu A, Chu D, Chen R (2009) Oxygen reduction on carbon supported Pt and PtRu catalysts in alkaline solutions. J Electroanal Chem 629:87–93
Macià MD, Campiña JM, Herrero E, Feliu JM (2004) On the kinetics of oxygen reduction on platinum stepped surfaces in acidic media. J Electroanal Chem 564:141–150
Kuzume A, Herrero E, Feliu JM (2007) Oxygen reduction on stepped platinum surfaces in acidic media. J Electroanal Chem 599:333–343
Grozovski V, Kasuk H, Nerut J, Härk E, Jäger R, Tallo I, Lust E (2015) Oxygen reduction at shape-controlled platinum nanoparticles and composite catalysts based on (100)Pt nanocubes on microporous–mesoporous carbon supports. ChemElectroChem 2:847–851
Paulus UA, Wokaun A, Scherer GG, Schmidt TJ, Stamenkovic V, Markovic NN, Ross PN (2002) Oxygen reduction on high surface area Pt-based alloy catalysts in comparison to well defined smooth bulk alloy electrodes. Electrochim Acta 47:3787–3798
Parthasarathy A, Srinivasan S, Appleby AJ, Martin CR (1992) Temperature dependence of the electrode kinetics of oxygen reduction at the platinum/nafion® Interface—a microelectrode investigation. J Electrochem Soc 139(9):2530–2537
Marek P, Velasco-Veléz JJ, Doll T, Sadowski G (2014) Compensation for the influence of temperature and humidity on oxygen diffusion in a reactive polymer matrix. J Sens Sen Syst 3:291–303
Daikhin LI, Kornyshev AA, Urbakh M (1998) Nonlinear Poisson-Boltzmann theory of a double layer at rough metal/electrolyte interface: a new look on the capacitance data on solid electrodes. J Chem Phys 108:1715–1723
Daikhin LI, Kornyshev AA, Urbakh M (1996) Double-layer capacitance on a rough metal surface. Phys Rev E 53:6192–6199
Lust E, Jänes A, Sammelselg V, Miidla P, Lust K (1998) Surface roughness of bismuth, antimony and cadmium electrodes. Electrochim Acta 44:373–383
Lust E, Jänes A, Sammelselg V, Miidla P (2000) Influence of charge density on the electrochemical surface roughness of cadmium electrode. Electrochim Acta 46:185–191
Lust E, Kallip S, Möller P, Jänes A, Sammelselg V, Miidla P, Väärtnõu M, Lust K (2003) Influence of surface charge density on the electrochemical surface “roughness” of Bi electrodes. J Electrochem Soc 150:E175–E184
Collier CP, Saykally RJ, Shiang JJ, Henrichs SE, Heath JR (1997) Reversible tuning of silver quantum dot monolayers through the metal-insluator transition. Science 277(5334):1978–1981
Shinagawa T, Garcia-Esparza AT, Takanabe K (2015) Insight on Tafel slopes from a microkinetic analysis of aqueous electrocatalysis for energy conversion. Scientific Reports 5(13801):1–21
Zhang J (Ed) (2008) PEM fuel cell electrocatalysts and catalyst layers fundamentals and applications, Springer-Verlag, London Limited pp 89–134
Gómez-Marín AM, Rizo R, Feliu JM (2013) Some reflections on the understanding of the oxygen reduction reaction at Pt(111). Beilstein J Nanotechnol 4:956–967
Perez J, Gonzalez ER, Ticianelli EA (1998) Oxygen electrocatalysis on thin porous coating rotating platinum electrodes. Electrochim Acta 44(8–9):1329–1339
Acknowledgements
This work was supported by the European Spallation Source Project: Estonian Partition in ESS Instrument design, development; building and application for scientific research: SLOKT12026T, the Estonian institutional research grant No. IUT20-13, the Estonian Centre of Excellence in Science: TK117T “High-technology Materials for Sustainable Development”, the European Regional Development Fund: TK141 “Advanced materials and high-technology devices for energy recuperation systems”, the Estonian Energy Technology Program: SLOKT10209T, the Materials Technology Project: SLOKT12180T, NAMUR “Nanomaterials—research and applications” (3.2.0304.12-0397), and by personal research grant No. PUT55. The authors would like to thank Dr. Karmen Lust for providing critical comments and English corrections of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
E. Härk, R. Jäger, P. Möller, T. Romann and E. Lust are members of ISE.
E. Härk and E. Lust are members of ECS.
Rights and permissions
About this article
Cite this article
Härk, E., Jäger, R., Tallo, I. et al. Influence of chemical composition and amount of intermixed ionomer in the catalyst on the oxygen reduction reaction characteristics. J Solid State Electrochem 21, 2079–2090 (2017). https://doi.org/10.1007/s10008-017-3521-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-017-3521-7