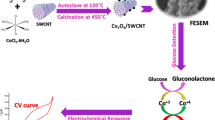
Abstract
The direct electron transfer of glucose oxidase (GOx) was achieved based on the immobilization of CdSe@CdS quantum dots on glassy carbon electrode by multi-wall carbon nanotubes (MWNTs)-chitosan (Chit) film. The immobilized GOx displayed a pair of well-defined and reversible redox peaks with a formal potential (E θ’) of −0.459 V (versus Ag/AgCl) in 0.1 M pH 7.0 phosphate buffer solution. The apparent heterogeneous electron transfer rate constants (k s) of GOx confined in MWNTs-Chit/CdSe@CdS membrane were evaluated as 1.56 s−1 according to Laviron's equation. The surface concentration (Γ*) of the electroactive GOx in the MWNTs-Chit film was estimated to be (6.52 ± 0.01) × 10−11 mol cm−2. Meanwhile, the catalytic ability of GOx toward the oxidation of glucose was studied. Its apparent Michaelis–Menten constant for glucose was 0.46 ± 0.01 mM, showing a good affinity. The linear range for glucose determination was from 1.6 × 10−4 to 5.6 × 10−3 M with a relatively high sensitivity of 31.13 ± 0.02 μA mM−1 cm−2 and a detection limit of 2.5 × 10−5 M (S/N=3).








Similar content being viewed by others
References
Fan C, Plaxco KW, Heeger AJ, Am J (2002) High-efficiency fluorescence quenching of conjugated polymers by proteins. J Am Chem Soc 124:5642–5643
Gao Q, Guo YY, Liu J, Yuan XQ, Qi HL, Zhang CX (2011) A biosensor prepared by co-entrapment of a glucose oxidase and a carbon nanotube within an electrochemically deposited redox polymer multilayer. Bioelectrochemistry 81:109–113
Kuznetsov BA, Shumakovich GP, Koroleva OV, Yaropolov AI (2001) On applicability of laccase as label in the mediated and mediatorless electroimmunoassay: effect of distance on the direct electron transfer between laccase and electrode. Biosens Bioelectron 16:73–84
Chen D, Li J (2006) Interfacial design and functionization on metalelectrodes through self-assembled monolayers. Surf Sci Rep 61:445–463
Lu X, Wen Z, Li J (2006) Hydroxyl-containing antimony oxide bromide nanorods combined with chitosan for biosensors. Biomaterials 27:5740–5747
Sun JY, Huang KJ, Zhao SF, Fan Y, Wu ZW (2011) Direct electrochemistry and electrocatalysis of hemoglobin on chitosan-room temperature ionic liquid-TiO2-graphene nanocomposite film modified electrode. Bioelectrochemistry 82:125–130
Yu WW, Qu L, Guo W, Peng X (2003) Experimental determination of the extinction coefficient of CdTe, CdSe, and CdS nanocrystals. Chem Mater 15:2854–2860
Peng X, Schlamp MC, Kadavanich AV, Alivisatos AP (1997) Epitaxial growth of highly luminescent CdSe/CdS core/shell nanocrystals with photostability and electronic accessibility. J Am Chem Soc 119:7019–7029
Li J, Wang YA, Guo WZ, Keay JC, Mishima TD, Johnson MB, Peng X (2003) Large-scale synthesis of nearlymonodisperse CdSe/CdS core/shell nanocrystals using air-stable reagents via successive ion layer adsorption and reaction. J Am Chem Soc 125:12567–12575
Munro AM, Plante IJ, Ng MS, Ginger DS (2007) Quantitative study of the effects of surface ligand concentration on CdSe nanocrystal photoluminescence. J Phys Chem C 111:6220–6227
Lu Q, Hu SS, Pang DW, He ZK (2005) Direct electrochemistry and electrocatalysis with hemoglobin in water-soluble quantum dots film on glassy carbon electrode. Chem Commun 20:2584–2585
Sajjadi S, Ghourchian H, Rahimi P (2011) Different behaviors of single and multi wall carbon nanotubes for studying electrochemistry and electrocatalysis of choline oxidase. Electrochim Acta 56:9542–9548
Chen H, Li R, Lin L, Guo GS, Lin JM (2010) Determination of L-ascorbic acid in human serum by chemiluminescence based on hydrogen peroxide-sodium hydrogen carbonate-CdSe/CdS. Talanta 81:1688–1696
Liu SQ, Ju HX (2003) Reagentless glucose biosensor based on direct electron transfer of glucose oxidase immobilized on colloidal gold modified carbon paste electrode. Biosens Bioelectron 19:177–183
Ju HX, Liu SQ, Ge B, Lisdat F, Scheller FW (2002) Electrochemistry of cytochrome c immobilized on colloidal gold modified carbon paste electrodes and its electrocatalytic activity. Electroanal 14:141–147
Liu Q, Lu XB, Li J, Yao X, Li JH (2007) Direct electrochemistry of glucose oxidase and electrochemical biosensing of glucose on quantum dots/carbon nanotubes electrodes. Biosens Bioelectron 22:3203–3209
Laviron E (1979) General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J Electroanal Chem 101:19–28
Huang YX, Zhang WJ, Xiao H, Li GX (2005) An electrochemical investigation of glucose oxidase at a CdS nanoparticles modified electrode. Biosens Bioelectron 21:817–821
Wang KQ, Yang H, Zhu L, Liao JH, Lu TH, Xing W, Xing SY, Lv Q (2009) Direct electrochemistry and electrocatalysis of glucose oxidase immobilized on glassy carbon electrode modified by Nafion and ordered mesoporous silica-SBA-15. J Mol Catal B:Enzym 58:194–198
Wang KQ, Yang H, Zhu L, Ma ZS, Xing SY, Lv Q, Liao JH, Liu CP, Xing W (2009) Direct electron transfer and electrocatalysis of glucose oxidase immobilized on glassy carbon electrode modified with Nafion and mesoporous carbon FDU-15. Electrochim Acta 54:4626–4630
Deng CY, Chen JH, Chen XL, Xiao CH, Nie LH, Yao SZ (2008) Direct electrochemistry of glucose oxidase and biosensing for glucose based on boron-doped carbon nanotubes modified electrode. Biosens Bioelectron 23:1272–1277
Li JW, Yu JJ, Zhao FQ, Zeng BZ (2007) Direct electrochemistry of glucose oxidase entrapped in nano gold particles-ionic liquid-N, N-dimethylformamide composite film on glassy carbon electrode and glucose sensing. Anal Chim Acta 587:33–40
Bourdillon C, Demaille C, Gueris J, Morioux J, Saveant JM (1993) A fully active monolayer enzyme electrode derivatized by antigen-antibody attachment. J Am Chem Soc 115:12264–12269
Zhang HF, Meng ZC, Wang Q, Zheng JB (2011) A novel glucose biosensor based on direct electrochemistry of glucose oxidase incorporated in biomediated gold nanoparticles–carbon nanotubes composite film. Sens Actuators B 158:23–27
Deng S, Jian G, Lei J, Hu Z, Ju H (2009) A glucose biosensor based on direct electrochemistry of glucose oxidase immobilized on nitrogen-doped carbon nanotubes. Biosens Bioelectron 25:373–377
Kamin RA, Wilson GS (1980) Rotating ring-dish enzyme electrode for biocatalysis kinetic studies and characterization of the immobilized enzyme layer. Anal Chem 52:1198–1205
Wang Y, Yuan R, Chaia YQ, Li WJ, Zhuo Y, YuanYL LJJ (2011) Direct electron transfer: electrochemical glucose biosensor based on hollow Pt nanosphere functionalized multiwall carbon nanotubes. J Mol Catal B:Enzym 71:146–151
Huang YX, Zhang WJ, Xiao H, Li GX (2005) An electrochemicalinvestigation of glucose oxidase at a CdS nanoparticles modified electrode. Biosens Bioelectron 21:817–821
Liu BH, Hu RQ, Deng JQ (1997) Characterization of immobilization of an enzyme in a modified Y zeolite matrix and its application to an amperometric glucose biosensor. Anal Chem 69:2343–2348
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos. 21175115), the Natural Science Foundation of Fujian province in China (2012J05031), the Zhangzhou Normal University scientific research projects (NO. SJ1117), and the Innovation Base Foundation for Graduate Students Education of Fujian Province.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huang, F., Wang, F., Feng, S. et al. Direct electrochemistry and electrochemical biosensing of glucose oxidase based on CdSe@CdS quantum dots and MWNT-modified electrode. J Solid State Electrochem 17, 1295–1301 (2013). https://doi.org/10.1007/s10008-012-1986-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-012-1986-y