Abstract
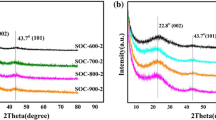
Micro- and mesoporous carbide-derived carbons (CDCs) were synthesised from TiC powder via a gas-phase reaction using HCl and Cl2 within the temperature range of 700–1,100 °C. Analysis of X-ray diffraction results show that TiC-CDCs consist mainly of graphitic crystallites. The first-order Raman spectra showed the graphite-like absorption peaks at ~1,577 cm−1 and the disorder-induced peaks at ~1,338 cm−1. The low-temperature N2 sorption experiments were performed, and specific surface areas up to 1,214 and 1,544 m2 g−1 were obtained for TiC-CDC (HCl) synthesised at T = 800 °C and TiC-CDC (Cl2) synthesised at T = 900 °C, respectively. For the TiC-CDC powders synthesised, a bimodal pore size distribution has been established with the first maximum in the region up to 1.5 nm and the second maximum from 2 to 4 nm. The energy-related properties of supercapacitors based on 1 M (C2H5)3CH3NBF4 in acetonitrile and TiC-CDC (Cl2) and TiC-CDC (HCl) as electrode materials were also investigated by cyclic voltammetry, impedance spectroscopy, galvanostatic charge/discharge and constant power methods. The specific energy, calculated at U = 3.0 V, are maximal for TiC-CDC (Cl2 800 °C) and TiC-CDC (HCl 900 °C), which are 43.1 and 31.1 W h kg−1, respectively. The specific power, calculated at cell potential U = 3.0 V, are maximal for TiC-CDC (Cl2 1,000 °C) and TiC-CDC (HCl 1,000 °C), which are 805.2 and 847.5 kW kg−1, respectively. The Ragone plots for CDCs prepared by using Cl2 or HCl are quite similar, and at high power loads, the TiC-CDC material synthesised using Cl2 at 900 °C, i.e. the material with optimal pore structure, delivers the highest power at constant energy.








Similar content being viewed by others
References
Gogotsi Y, Nikitin A, Ye H, Zhou W, Fischer JE, Yi B, Foley HC, Barsoum MW (2003) Nanoporous carbide-derived carbon with tunable pore size. Nat Mater 2:591–594
Jänes A, Permann L, Arulepp M, Lust E (2004) Electrochemical characteristics of nanoporous carbide-derived carbon materials in non-aqueous electrolyte solutions Electrochem Commun 6:313–318
Yushin G, Nikitin A, Gogotsi Y (2006) Carbide-derived carbon. In: Gogotsi Y (ed) Nanomaterials handbook. CRC, Boca Raton, pp 239–282
Batisse N, Guérin K, Dubois M, Hamwi A (2011) The synthesis of microporous carbon by the fluorination of titanium carbide. Carbon 49:2998–3009
Cambaz ZG, Yushin GN, Gogotsi Y (2006) Formation of carbide-derived carbon on beta-silicon carbide whiskers. J Am Ceram Soc 89:509–514
Presser V, Heaon M, Gogotsi Y (2011) From Porous Networks to Nanotubes and Graphene. Adv Funct Mater 21:810–833
Chmiola J, Yushin G, Gogotsi Y, Portet C, Simon P, Taberna PL (2006) Anomalous increase in carbon capacitance at pore sizes less than 1 nanometer. Science 313:1760–1763
Jänes A, Thomberg T, Lust E (2007) Synthesis and characterisation of nanoporous carbide-derived carbon by chlorination of vanadium carbide. Carbon 45:2717–2722
Jänes A, Thomberg T, Kurig H, Lust E (2009) Nanoscale fine-tuning of porosity of carbide-derived carbon prepared from molybdenum carbide. Carbon 47:23–29
Gogotsi Y et al (2005) Tailoring of Nanoscale Porosity in Carbide-Derived Carbons for Hydrogen Storage. J Am Chem Soc 127:160006
Yushin G et al (2006) Mesoporous carbide-derived carbon with porosity tuned for efficient adsorption of cytokines. Biomaterials 27:5755–5762
Laheäär A, Jänes A, Lust E (2011) Electrochemical properties of carbide-derived carbon electrodes in non-aqueous electrolytes based on different Li-salts. Electrochim Acta 56:9048–9055
Klug HP, Alexander LE (1974) X-ray diffraction procedures. Wiley, New York, pp 667–668
Nemanich RJ, Solin SA (1979) First- and second-order Raman scattering from finite-size crystals of graphite. Phys Rev B 20:392–401
Tuinstra F, Koenig JL (1970) Raman Spectrum of Graphite. J Chem Phys 53:1126–1130
Ferrari AC, Robertson J (2000) Interpretation of Raman spectra of disordered and amorphous carbon. Phys Rev B 61:14095–14107
Ferrari AC (2007) Raman spectroscopy of graphene and graphite: disorder, electron-phonon coupling, doping and nonadiabatic effects. Solid State Commun 143:47–57
Dresselhaus MS, Dresselhaus G, Hofmann M (2007) The big picture of Raman scattering in carbon nanotubes. Vib Spectrosc 45:71–81
Babu VS, Seehra MS (1996) Modeling of disorder and x-ray diffraction in coal-based graphitic carbons. Carbon 34:1259–1265
Williams DB, Carter CB (1996) Transmission electron microscopy. Plenum, New York
Brunauer S, Emmett PH, Teller E (1938) Adsorption of gases in multimolecular layers. J Am Chem Soc 6:309–319
Rouquerol F, Rouquerol J, Sing K (1999) Adsorption by powders and porous solids: Principles, methodology and applications. Academic, London, pp 165–189
Ravikovitch PI, Vishnyakov A, Neimark AV (2001) Density functional theories and molecular simulations of adsorption and phase transitions in nanopores. Phys Rev E 64:011602
Ravikovitch PI, Neimark AV (2001) Characterization of Nanoporous Materials from Adsorption and Desorption Isotherms. Colloid Surf A 187:11–21
Lastoskie C, Gubbins KE, Quirke N (1993) Pore size distribution analysis of microporous carbons: a density functional theory approach. J Phys Chem 97:4786–4796
Lastoskie C, Gubbins KE, Quirke N (1993) Pore size heterogeneity and the carbon slit pore: A density functional theory model Langmuir 9:2693–2702
Chmiola J, Yushin G, Dash R, Gogotsi Y (2006) Effect of pore size and surface area of carbide derived carbons on specific capacitance. J Power Sources 158:765–772
Oschatz M, Kockrick E, Rose M, Borchardt L, Klein N, Senkovska I, Freudenberg T, Korenblit Y, Yushin G, Kaskel S (2010) A cubic ordered, mesoporous carbide-derived carbon for gas and energy storage applications. Carbon 48:3987–3992
Thomberg T, Jänes A, Lust E (2010) Energy and power performance of electrochemical double-layer capacitors based on molybdenum carbide derived carbon. Electrochim Acta 55:3138–3143
Kim YJ, Masutzawa Y, Ozaki S, Endo M, Dresselhaus MS (2004) PVDC-based carbon material by chemical activation and its application to nonaqueous EDLC. J Electrochem Soc 151:E199–E205
Pell WG, Conway BE (2001) Voltammetry at a de Levie brush electrode as a model for electrochemical supercapacitor behavior. J Electroanal Chem 500:121–133
Gileadi E (1993) Electrode kinetics for chemists, chemical engineering and materials scientists. VCH, New York
Tallo I, Thomberg T, Jänes A, Kontturi K, Lust E (2011) Nanostructured carbide-derived carbon synthesized by chlorination of tungsten carbide. Carbon 49:4427–4433
Salitra G, Soffer A, Eliad L, Cohen Y, Aurbach D (2000) Carbon electrodes for double-layer capacitors - I. Relations between ion and pore dimensions. J Electrochem Soc 147:2486–2493
Eikerling M, Kornyshev AA, Lust E (2005) Optimized structure of nanoporous carbon-based double-layer capacitors. J Electrochem Soc 152:E24–E33
Chmiola J, Largeot C, Taberna PL, Simon P, Gogotsi Y (2008) Desolvation of ions in subnanometer pores and its effect on capacitance and double-layer theory. Angew Chem Int Ed 47:3392–3395
Korenblit Y, Rose M, Kockrick E, Borchardt L, Kvit A, Kaskel S, Yushin G (2010) High-Rate Electrochemical Capacitors Based on Ordered Mesoporous Silicon Carbide-Derived Carbon. ACS Nano 4:1337–1344
Thomberg T, Jänes A, Lust E (2009) Energy and power performance of vanadium carbide derived carbon electrode materials for supercapacitors. J Electroanal Chem 630:55–62
Acknowledgments
This work was supported in part by the Estonian Ministry of Education and Research (project SF0180002s08), by the Estonian Centre of Excellence in Science: High Technology Materials for Sustainable Development, by the graduate school “Functional Materials and Technologies”, receiving funding from the European Social Fund under project 1.2.0401.09-0079 in Estonia and by the Estonian Science Foundation under project no. 8172. Prof. K. Kirsimäe from the Institute of Ecology and Geography and Dr. I. Sildos from the Institute of Physics at the University of Tartu are thanked for the help with the XRD and Raman studies of carbon samples, respectively.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tallo, I., Thomberg, T., Kurig, H. et al. Supercapacitors based on carbide-derived carbons synthesised using HCl and Cl2 as reactants. J Solid State Electrochem 17, 19–28 (2013). https://doi.org/10.1007/s10008-012-1850-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-012-1850-0