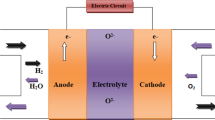
Abstract
Following previous surveys of the solid electrolyte ceramics and electrode reaction mechanisms in solid oxide fuel cells, this review is focused on the comparative analysis of electrochemical performance, thermal expansion, oxygen ionic and electronic transport, and durability-determining factors in the major groups of electrode materials. The properties of mixed-conducting oxide phases with perovskite-related and fluorite structures, ceramic–metal and oxide composites, and catalytically active additives are briefly discussed, with emphasis on the approaches and findings reported during the last 10–15 years. The performance of conventional and alternative electrode materials in the cells with ZrO2-, CeO2-, LaGaO3-, and La10Si6O27-based electrolytes is appraised in the context of potential optimization strategies. Particular attention is centered on the cathode and anode compositions providing maximum electrochemical activity and stability and on the critical aspects relevant for electrode microstructure engineering.





















Similar content being viewed by others
References
Minh NQ, Takahashi T (1995) Science and technology of ceramic fuel cells. Elsevier, Amsterdam
Singhal SC (2000) Mater Res Soc Bull 25:16
Yamamoto O (2000) Electrochim Acta 45:2423
Steele BCH (2001) J Mater Sci 36:1053
Huijsmans JPP (2001) Curr Opin Solid State Mat Sci 5:317
Gorte RJ (2005) AIChE J 51:2377
De Bruijn F (2005) Green Chem 7:132
Kendall K (2005) Int Mater Rev 50:257
Sun C, Stimming U (2007) J Power Sources 171:247
La O’ GJ, In HJ, Grumlin E, Barbastatsis G, Shao-Horn Y (2007) Int J Energy Res 31:548
Beckel D, Bieberle-Hütter A, Harvey A, Infortuna A, Muecke UP, Prestat M, Rupp JLM, Gauckler LJ (2007) J Power Sources 171:325
Lessing PA (2007) J Mater Sci 42:3465
McEvoy AJ, Rambert S, Widmer S (1996) Nanostructure, defect chemistry and operating protocols influence SOFC performance. In: Thorstensen, B (eds) Proc 2nd Eur Solid Oxide Fuel Cell Forum (Oslo, May 1996). vol. 1. European SOFC Forum, Oberrohrdorf, Switzerland, pp 599–606
Bronin DI, Kuzin BL, Yaroslavtsev IY, Bogdanovich NM (2006) J Solid State Electrochem 10:651
Lust E, Möller P, Kivi I, Nurk G, Kallip S (2005) J Solid State Electrochem 9:882
Horita T, Kishimoto H, Yamaji K, Xiong Y, Sakai N, Brito ME, Yokokawa H (2006) Solid State Ionics 177:1941
Dalslet B, Blennow P, Hendriksen PV, Bonanos N, Lybye D, Mogensen M (2006) J Solid State Electrochem 10:547
Etsell TH, Flengas SN (1970) Chem Rev 70:339
Chebotin VN, Perfiliev MV (1978) Electrochemistry of solid electrolytes. Technical Information Center, US Department of Energy, Oak Ridge
Kinoshita K (1992) Electrochemical oxygen technology. Wiley, New York
Bouwmeester HJM, Burgraaf AJ (1996) Dense ceramic membranes for oxygen separation. In: Burggraaf, AJ, Cot, L (eds) Fundamentals of inorganic membrane science and technology. Elsevier, Amsterdam, pp 435–528
Riess I, Schoonman J (1997) Electrodics. In: Gellings, PJ, Bouwmeester, HJM (eds) CRC handbook of solid state electrochemistry. CRC, Boca Raton, pp 269–294
Palguev SF, Gilderman VK, Zemtsov VI (1990) High-temperature oxide electronic conductors for electrochemical devices. Nauka, Moscow
Baker R, Guindet J, Kleitz M (1997) J Electrochem Soc 144:2427
Kharton VV, Yaremchenko AA, Naumovich EN (1999) J Solid State Electrochem 3:303
Adler SB (2004) Chem Rev 104:4791
Fergus JW (2006) Solid State Ionics 177:1529
Bove R, Ubertini S (2006) J Power Sources 159:543
Jiang SP (2007) J Solid State Electrochem 11:93
Kharton VV, Naumovich EN, Vecher AA (1999) J Solid State Electrochem 3:61
Blum L, Meulenberg WA, Nabielek H, Steinberger-Wilckens R (2005) Int J Appl Ceram Technol 2:482
Gaiduk YS, Kharton VV, Naumovich EN, Nikolaev AV, Samokhval VV (1994) Inorg Mater 30:1360
Takeda Y, Tu HY, Sakaki H, Watanabe S, Imanishi N, Yamamoto O, Phillipps MB, Sammes NM (1997) J Electrochem Soc 144:2810
Takeda Y, Sakaki Y, Ichikawa T, Imanishi N, Yamamoto O, Mori M, Abe T (1994) Solid State Ionics 72:257
Ishihara T, Kudo T, Matsuda H, Takita Y (1995) J Electrochem Soc 142:1519
Hashimoto S, Iwahara H (2000) J Electroceram 4:225
Huang X, Liu J, Lu Z, Liu W, Pei L, He T, Liu Z, Su W (2000) Solid State Ionics 130:195
Schachtner R, Ivers-Tiffée E, Weppner W, Männer R, Wersing W (1995) Ionics 1:63
Al Daroukh M, Vashook VV, Ullmann H, Tietz F, Arual Raj I (2003) Solid State Ionics 158:141
Siebert E, Hammouche A, Kleitz M (1995) Electrochim Acta 40:1741
Lee HY, Cho WS, Oh SM, Wiemhöfer H-D, Göpel W (1995) J Electrochem Soc 142:2659
Yasumoto K, Mori N, Mizusaki J, Tagawa H, Dokiya M (2001) J Electrochem Soc 148:A105
Sakaki Y, Takeda Y, Kato A, Imanishi N, Yamamoto O, Hattori M, Iio M, Esaki Y (1999) Solid State Ionics 118:187
Kharton VV, Nikolaev AV, Naumovich EN, Vecher AA (1995) Solid State Ionics 81:201
Huebner W, Reed DM, Anderson HU (1997) Structure–property relationships in solid oxide fuel cells. In: Stimming, U, Singhal, SC, Tagawa, H, Lehnert, W (eds) SOFC V. The Electrochemical Society, Pennington, NJ, pp 411–420
Rim H-R, Jeung S-K, Jung E, Lee J-S (1998) Mater Chem Phys 52:54
van Roosmalen JAM, Cordfunke EHP (1992) Solid State Ionics 52:303
van Heuveln FH, Bouwmeester HJM (1997) J Electrochem Soc 144:134
Kamata H, Hosaka A, Mizusaki J, Tagawa H (1998) Solid State Ionics 106:237
Tikhonovich VN, Kharton VV, Naumovich EN, Savitsky AA (1998) Solid State Ionics 106:197
Takeda Y, Kanno R, Noda M, Tomida Y, Yamamoto O (1987) J Electrochem Soc 134:2656
Pena MA, Fierro JLG (2001) Chem Rev 101:1981
Jiang SP (2003) J Power Sources 124:390
Mitterdorfer A, Gauckler LJ (1998) Solid State Ionics 111:185
Yokokawa H, Sakai N, Kawada T, Dokiya M (1993) Chemical thermodynamic stability of the interface. In: Badwal, SPS, Bannister, MJ, Hannink, RHJ (eds) Science and technology of Zirconia V. The Australian Ceramic Society, Technomic Publ, Melbourne, Australia, pp 752–763
Weber A, Männer R, Waser R, Ivers-Tiffée E (1996) Denki Kagaku 64:582
Caillol N, Pijolat M, Siebert E (2007) Appl Surf Sci 253:4641
Holc J, Kuščer D, Hrovat M, Bernik S, Kolar D (1997) Solid State Ionics 95:259
Phillipps MB, Sammes NM, Yamamoto O (1999) Solid State Ionics 123:131
Marozau IP, Kharton VV, Viskup AP, Frade JR, Samakhval VV (2006) J Eur Ceram Soc 26:1371
Barbucci A, Viviani M, Panizza M, Delucchi M, Cerisola G (2005) J Appl Electrochem 35:399
Leng YJ, Chan SH, Khor KA, Jiang SP (2006) J Solid State Electrochem 10:339
Godoi GS, de Souza DPF (2007) Mater Sci Eng B 140:90
Chen K, Lu Z, Ai N, Chen X, Hu J, Huang X, Su W (2007) J Power Sources 167:84
Kim J-H, Song R-H, Kim JH, Lim T-H, Sun Y-K, Shin D-R (2007) J Solid State Electrochem 11:1385
Hagiwara A, Hobara N, Takizawa K, Sato K, Abe H, Naito M (2007) Solid State Ionics 178:1123
Gong W, Gopalan S, Pal UB (2004) J Mater Eng Perform 13:274
Goodenough JB, Zhou J-S (2001) Transport properties. In: Goodenough, JB (eds) Localized to itinerant electronic transition in perovskite oxides. Springer, Berlin, pp 17–114
McCammon C (1996) Phase Transit 58:1
Oleynikov NN, Ketsko VA (2004) Russ J Inorg Chem 49:1
Gauckler LJ, Beckel D, Buergler BE, Jud E, Muecke UP, Prestat M, Rupp LJM, Richter J (2004) Chimia 58:837
Mai A, Haanappel VAC, Uhlenbruck S, Tietz F, Stöver D (2005) Solid State Ionics 176:1341
Lashtabeg A, Skinner SJ (2006) J Mater Chem 16:1
Liu Y, Tan X, Li K (2006) Catal Rev 48:145
Kharton VV, Yaremchenko AA, Shaula AL, Viskup AP, Marques FMB, Frade JR, Naumovich EN, Casanova JR, Marozau IP (2004) Defect Diffus Forum 226–228:141
Patrakeev MV, Leonidov IA, Kozhevnikov VL, Kharton VV (2004) Solid State Sci 6:907
Markov AA, Patrakeev MV, Kharton VV, Pivak YV, Leonidov IA, Kozhevnikov VL (2007) Chem Mater 19:3980
Kovalevsky AV, Kharton VV, Snijkers FMM, Cooymans JFC, Luyten JJ, Marques FMB (2007) J Membrane Sci 301:238
Patrakeev MV, Bahteeva JA, Mitberg EB, Leonidov IA, Kozhevnikov VL, Poeppelmeier KR (2003) J Solid State Chem 172:219
Tsipis EV, Patrakeev MV, Kharton VV, Yaremchenko AA, Mather GC, Shaula AL, Leonidov IA, Kozhevnikov VL, Frade JR (2005) Solid State Sci 7:355
Kharton VV, Patrakeev MV, Waerenborgh JC, Kovalevsky AV, Pivak YV, Gaczyński P, Markov AA, Yaremchenko AA (2007) J Phys Chem Solids 68:355
Jennings AJ, Skinner SJ, Helgason O (2003) J Solid State Chem 175:207
Lein HL, Wiik K, Grande T (2006) Solid State Ionics 177:1795
Søgaard M, Hendriksen PV, Mogensen M (2007) J Solid State Chem 180:1489
Zajac W, Swierczek K, Molenda J (2007) J Power Sources 173:675
Kharton VV, Yaremchenko AA, Patrakeev MV, Naumovich EN, Marques FMB (2003) J Eur Ceram Soc 23:1417
Kharton VV, Shaulo AL, Viskup AP, Avdeev M, Yaremchenko AA, Patrakeev MV, Kurbakov AI, Naumovich EN, Marques FMB (2002) Solid State Ionics 150:229
Park CY, Jacobson AJ (2005) Solid State Ionics 176:2671
Tsipis EV, Kharton VV, Vyshatko NP, Frade JR, Marques FMB (2005) Solid State Sci 7:257
Kharton VV, Figueiredo FM, Navarro L, Naumovich EN, Kovalevsky AV, Yaremchenko AA, Viskup AP, Carneiro A, Marques FMB, Frade JR (2001) J Mater Sci 36:1105
Kammer K, Mikkelsen L, Bilde-Sørensen JB (2006) J Solid State Electrochem 10:1432
Hashimoto S, Kammer K, Larsen PH, Poulsen FW, Mogensen M (2005) Solid State Ionics 176:1013
Prado F, Gurunathan K, Manthiram A (2002) J Mater Chem 12:2390
Shaula AL, Pivak YV, Waerenborgh JC, Gaczyński P, Yaremchenko AA, Kharton VV (2006) Solid State Ionics 177:2923
Kindermann L, Das D, Nickel H, Hilpert K (1996) Solid State Ionics 89:215
Kindermann L, Das D, Nickel H, Hilpert K, Appel CC, Poulsen FW (1997) J Electrochem Soc 144:717
Anderson MD, Stevenson JW, Simner SP (2004) J Power Sources 129:188
Kostogloudis GC, Tsiniarakis G, Ftikos C (2000) Solid State Ionics 135:529
Simner SP, Shelton JP, Anderson MD, Stevenson JW (2003) Solid State Ionics 161:11
Hrovat M, Holc J, Bernik S, Makovec D (1998) Mater Res Bull 33:1175
Kharton VV, Kovalevsky AV, Viskup AP, Shaula AL, Figueiredo FM, Naumovich EN, Marques FMB (2003) Solid State Ionics 160:247
Sakai N, Horita T, Yamaji K, Brito ME, Yokokawa H, Kawakami A, Matsuoka S, Watanabe N, Ueno A (2006) J Electrochem Soc 153:A621
Shaula AL, Kharton VV, Marques FMB (2004) J Eur Ceram Soc 24:2631
Shaula AL, Kharton VV, Marques FMB, Kovalevsky AV, Viskup AP, Naumovich EN (2006) J Solid State Electrochem 10:28
Sakai N, Kishimoto H, Yamaji K, Horita T, Brito ME, Yokokawa H (2007) J Electrochem Soc 154:B1331
Wan J-H, Yan J-Q, Goodenough JB (2005) J Electrochem Soc 152:A1511
Ullmann H, Trofimenko N, Tietz F, Stöver D, Ahmad-Khanlou A (2000) Solid State Ionics 138:79
Hayashi H, Suzuki M, Inaba H (2000) Solid State Ionics 128:131
Kostogloudis GC, Ftikos C (1999) Solid State Ionics 126:143
Riza F, Ftikos C, Tietz F, Fischer W (2001) J Eur Ceram Soc 21:1769
Jiang SP (2002) Solid State Ionics 146:1
Bae J-M, Steele BCH (1998) Solid State Ionics 106:247
Kostogloudis GC, Ftikos C, Ahmad-Khanlou A, Naoumidis A, Stöver D (2000) Solid State Ionics 134:127
Stevenson JW, Armstrong TR, Carneim RD, Pederson LR, Weber WJ (1996) J Electrochem Soc 143:2722
Petrov AN, Cherepanov VA, Zuev AYu (2006) J Solid State Electrochem 10:517
Huang K, Lee HY, Goodenough JB (1998) J Electrochem Soc 145:3220
Takeda Y, Ueno H, Imanishi N, Yamamoto O, Sammes N, Phillipps MB (1996) Solid State Ionics 86–88:1187
Lee HY, Oh SM, Seo IY, Rocholl F, Wiemhöfer HD (1997) The cathodic activity and interfacial stability of Y0.8Ca0.2Co1 − x Fe x O3/YSZ electrodes. In: Stimming, U, Singhal, SC, Tagawa, H, Lehnert, W (eds) SOFC V. The Electrochemical Society, Pennington, NJ, pp 520–529
Naumovich EN, Kharton VV, Samokhval VV, Kovalevsky AV (1997) Solid State Ionics 93:95
Ishihara T, Honda M, Shibayama T, Furutani H, Takita Y (1998) Ionics 4:395
Chen F, Xia C, Liu M (2001) Chem Lett 30:1032
Uchida H, Arisaka S, Watanabe M (2002) J Electrochem Soc 149:A13
Uhlenbruck S, Tietz F (2004) Mater Sci Eng B 107:277
Lust E, Möller P, Kivi I, Nurk G, Kallip S (2005) J Solid State Electrochem 9:882
Kharton VV, Tsipis EV, Yaremchenko AA, Marozau IP, Viskup AP, Frade JR, Naumovich EN (2006) Mater Sci Eng B 134:80
Taskin AA, Lavrov AN, Ando Y (2005) Appl Phys Lett 86:091910
Streule S, Podlesnyak A, Sheptyakov D, Pomyakushina E, Stingaciu M, Conder K, Medarde M, Patrakeev M, Leonidov I, Kozhevnikov V, Mesot J (2006) Phys Rev B 73:094203
Tarascon A, Skinner SJ, Chater RJ, Hernandez-Ramirez F, Kilner JA (2007) J Mater Chem 17:3175
Tarascon A, Morata A, Dezanneau G, Skinner SJ, Kilner JA, Estrade S, Hernandez-Ramirez F, Peiro F, Morante JR (2007) J Power Sources 174:255
Valldor M (2004) Solid State Sci 6:251
Tsipis EV, Kharton VV, Frade JR, Nunez P (2005) J Solid State Electrochem 9:547
Hao H, Cui J, Chen C, Pan L, Hu J, Hu X (2006) Solid State Ionics 177:631
Lee KT, Manthiram A (2006) Chem Mater 18:1621
Kawada T, Sase M, Kudo M, Yashiro K, Sato K, Mizusaki J, Sakai N, Horita T, Yamaji K, Yokokawa H (2006) Solid State Ionics 177:3081
Tsipis EV, Kharton VV, Frade JR (2006) Solid State Ionics 177:1823
Petric A, Huang P, Tietz F (2000) Solid State Ionics 135:719
Kharton VV, Naumovich EN, Vecher AA, Nikolaev AV (1995) J Solid State Chem 120:128
Ishihara T, Fukui S, Nishiguchi H, Takita Y (2002) Solid State Ionics 152–153:609
Ohbayashi H, Kudo T, Gejo T (1974) Jpn J Appl Phys 13:1
Kharton VV, Naumovich EN, Zhuk PP, Demin AK, Nikolaev AV (1992) Russ J Electrochem 28:1376
Morin F, Trudel G, Denos Y (1997) Solid State Ionics 96:129
Poznyak SK, Kharton VV, Frade JR, Yaremchenko AA, Tsipis EV, Yakovlev SO, Marozau IP (2008) J Solid State Electrochem 12:15
Bucher E, Sitte W, Rom I, Rapst I, Grogger W, Hofer F (2002) Solid State Ionics 152–153:417
van Doorn RHE, Burggraaf AJ (2000) Solid State Ionics 128:65
Svarcova S, Wiik K, Tolchard J, Bouwmeester HJM, Grande T (2008) Solid State Ionics 178:1787
Vente JF, McIntosh S, Haije WG, Bouwmeester HJM (2006) J Solid State Electrochem 10:581
Baumann FS, Fleig J, Cristiani G, Stuhlhofer B, Habermeier H-U, Maier J (2007) J Electrochem Soc 154:B931
Peña-Martinez J, Marrero-Lopez D, Perez-Coll D, Ruiz-Morales JC, Nuñez P (2007) Electrochim Acta 52:2950
Shao Z, Xiong G, Tong J, Dong H, Yang W (2001) Sep Purif Technol 25:419
Vente JF, Haije WG, Rak ZS (2006) J Membrane Sci 276:178
Zeng P, Chen Z, Zhou W, Gu H, Shao Z, Liu S (2007) J Membrane Sci 291:148
Fisher CAJ, Yoshiya M, Iwamoto Y, Ishii J, Asanuma M, Yabuta K (2007) Solid State Ionics 177:3425
Yan A, Cheng M, Dong Y, Yang W, Maragou V, Song S, Tsiarakas P (2006) Appl Catal B 66:64
Simner SP, Shelton JP, Anderson MD, Stevenson JW (2003) Solid State Ionics 161:11
Chen W, Wen T, Nie H, Zheng R (2003) Mater Res Bull 38:1319
Yokokawa H, Sakai N, Horita T, Yamaji K, Brito ME, Kishimoto H (2008) J Alloys Compd 452:41
Bronin DI, Kuzin BL, Sokolova YV, Polyakova NV (2000) Russ J Appl Chem 73:1557
Kharton VV, Naumovich EN, Yaremchenko AA, Marques FMB (2001) J Solid State Electrochem 5:160
Kuzin BL, Bogdanovich NM, Bronin DI, Yaroslavtsev IY, Vdovin GK, Kotov YA, Bagazeev AV, Medvedev AI, Murzakaev AM, Timoshenkova OP, Stolts AK (2007) Russ J Electrochem 43:920
Zhu XD, Sun KN, Zhang NQ, Chen XB, Wu LJ, Jia DC (2007) Electrochem Commun 9:431
Zhou W, Shao Z, Ran R, Chen Z, Zeng P, Gu H, Jin W, Xu N (2007) Electrochim Acta 52:6297
Serra JM, Vert VB, Betz M, Haanappel VAC, Meulenberg WA, Tietz F (2008) J Electrochem Soc 155:B207
Ishihara T, Honda M, Nishiguchi H, Takita Y (1997) Solid oxide fuel cell operable at decreased temperature using LaGaO3 perovskite oxide electrolyte. In: Stimming, U, Singhal, SC, Tagawa, H, Lehnert, W (eds) SOFC V. The Electrochemical Society, Pennington, NJ, pp 301–310
Anderson HU (1992) Solid State Ionics 52:33
van Hassel BA, Kawada T, Sakai N, Yokokawa H, Dokiya M, Bouwmeester HJM (1993) Solid State Ionics 66:295
Tu HY, Takeda Y, Imanishi N, Yamamoto O (1999) Solid State Ionics 117:277
Kharton VV, Naumovich EN, Samokhval VV (1997) Solid State Ionics 99:269
Steele BCH (1995) Solid State Ionics 75:157
Endo A, Wada S, Wen C, Komiyama H, Yamada K (1998) J Electrochem Soc 145:L35
Fleig J (2002) J Power Sources 105:228
Fleig J, Maier J (2004) J Eur Ceram Soc 24:1343
Nakamura T, Petzow G, Gauckler LJ (1979) Mater Res Bull 14:649
Cheng J, Navrotsky A, Zhou XD, Anderson HU (2005) J Mater Res 20:191
Chiba R, Yoshimura F, Sakurai Y (1999) Solid State Ionics 124:281
Zinkevich M, Aldinger F (2004) J Alloys Compd 375:147
Bannikov DO, Cherepanov VA (2006) J Solid State Chem 179:2721
Amow G, Skinner SJ (2006) J Solid State Electrochem 10:538
Amow G, Davidson IJ, Skinner SJ (2006) Solid State Ionics 177:1205
Tsipis EV, Patrakeev MV, Waerenborgh JC, Pivak YV, Markov AA, Gaczyński P, Naumovich EN, Kharton VV (2007) J Solid State Chem 180:1902
Kovalevsky AV, Kharton VV, Yaremchenko AA, Pivak YV, Tsipis EV, Yakovlev SO, Markov AA, Naumovich EN, Frade JR (2007) J Electroceram 18:205
Gaiduk YS, Kharton VV, Naumovich EN, Samokhval VV (1994) Inorg Mater 30:759
Chiba R, Yoshimura F, Sakurai Y (2002) Solid State Ionics 152–153:575
Kiselev EA, Proskurnina NV, Voronin VI, Cherepanov VA (2007) Inorg Mater 43:167
Hashimoto S, Kammer K, Poulsen FW, Mogensen M (2007) J Alloys Compd 428:256
Kharton VV, Viskup AP, Naumovich EN, Tikhonovich VN (1999) Mater Res Bull 34:1311
Kharton VV, Viskup AP, Yaremchenko AA, Baker RT, Gharbage B, Mather GC, Figueiredo FM, Naumovich EN, Marques FMB (2000) Solid State Ionics 132:119
Yakovlev SO, Kharton VV, Naumovich EN, Zekonyte J, Zaporojtchenko V, Kovalevsky AV, Yaremchenko AA, Frade JR (2006) Solid State Sci 8:1302
Boehm E, Bassat J-M, Steil MC, Dordor P, Mauvy F, Grenier J-C (2003) Solid State Sci 5:973
Vashook V, Yushkevich I, Kokhanovsky L, Makhnach L, Tolochko S, Kononyuk I, Ullmann H, Altenburg H (1999) Solid State Ionics 119:23
Tsipis EV, Naumovich EN, Patrakeev MV, Waerenborgh JC, Pivak YV, Gaczyński P, Kharton VV (2007) J Phys Chem Solids 68:1443
Skinner SJ, Kilner JA (2000) Solid State Ionics 135:709
Kharton VV, Viskup AP, Kovalevsky AV, Naumovich EN, Marques FMB (2001) Solid State Ionics 143:337
Tsipis EV, Naumovich EN, Shaula AL, Patrakeev MV, Waerenborgh JC, Kharton VV (2007) Solid State Ionics 179:57
Mauvy F, Lalanne C, Bassat J-M, Grenier J-C, Zhao H, Dordor Ph, Stevens P (2005) J Eur Ceram Soc 25:2669
Kharton VV, Kovalevsky AV, Avdeev M, Tsipis EV, Patrakeev MV, Yaremchenko AA, Naumovich EN, Frade JR (2007) Chem Mater 19:2027
Kharton VV, Tsipis EV, Yaremchenko AA, Frade AA (2004) Solid State Ionics 166:327
Kim GT, Wang S, Jacobson AJ, Yuan Z, Chen C (2007) J Mater Chem 17:1316
Amow G, Whitfield PS, Davidson IJ, Hammond RP, Munnings CN, Skinner SJ (2004) Ceram Int 30:1635
Wan J, Goodenough JB, Zhu JH (2007) Solid State Ionics 178:281
Laberty C, Zhao F, Swider-Lyons KE, Virkar AV (2007) Electrochem Solid State Lett 10:B170
Schenck P (ed) (1998) Phase equilibria diagrams. CD-Rom database. The American Ceramic Society, Westerville, OH
Wong NGW, Cook LP (1992) AIChE Symp Ser 88:11
Osamura K, Zhang W (1993) Mater Res Adv Tech 84:522
Singh KK, Morris DE, Sinha APB (1994) Physica C 231:377
Kale GM, Kumar RV, Fray DJ (1996) Solid State Ionics 86–88:1421
Kononyuk IF, Tikhonova LA, Makhnach LV, Zhavnerko GK (1983) Neorg Mater 19:934
Berenov A, Wei J, Wood H, Rudkin R, Atkinson A (2007) J Solid State Electrochem 11:482
Bochkov DM, Kharton VV, Kovalevsky AV, Viskup AP, Naumovich EN (1999) Solid State Ionics 120:281
Opila EJ, Tuller HL, Wuensch BJ, Maier J (1993) J Am Ceram Soc 76:2363
Routbort JL, Rothman SJ, Flandermeyer BK, Nowicki LJ, Baker JE (1988) J Mater Res 3:116
Mazo GN, Savvin SN, Abakumov AM, Hadermann J, Dobrovol’skii Yu A, Leonova LS (2007) Russ J Electrochem 43:436
Patrakeev MV, Leonidov IA, Kozhevnikov VL, Tsidilkovskii VI, Demin AK, Nikolaev AV (1993) Solid State Ionics 66:61
Mozhaev AP, Mazo GN, Galkin AA, Khramova NV (1996) Russ J Inorg Chem 41:881
Chang CL, Lee TC, Huang TJ (1998) J Solid State Electrochem 2:291
Mizusaki J, Tagawa H, Katou M, Hirano K, Sawata A, Tsuneyoshi K (1991) Electrochemical properties of some perovskite-type oxides as oxygen gas electrodes on yttria stabilized zirconia. In: Grosz, F, Zegers, P, Singhal, SC, Yamamoto, O (eds) SOFC II. The Electrochemical Society, Pennington, NJ, pp 487–494
Li Q, Zhao H, Huo L, Sun L, Cheng X, Grenier J-C (2007) Electrochem Commun 9:1508
Murray EP, Barnett SA (2001) Solid State Ionics 143:265
Zhao H, Huo L, Gao S (2004) J Power Sources 125:149
Xia C, Rauch W, Chen F, Liu M (2002) Solid State Ionics 149:11
Hart NT, Brandon NP, Day MJ, Lapena-Rey N (2002) J Power Sources 106:42
Imahara K, Jacobson CP, Visco SJ, De Jonghe LC (2005) Solid State Ionics 176:451
Jiang SP (2006) Mater Sci Eng A 418:199
Wang W, Gross MD, Vohs JM, Gorte RJ (2007) J Electrochem Soc 154:B439
Sunde S (1995) J Electrochem Soc 142:L50
Kenjo T, Nishiya M (1992) Solid State Ionics 57:295
Wang S, Jiang Y, Zhang Y, Yan J, Li W (1998) J Electrochem Soc 145:1932
Kim JD, Kim GD, Moon JW, Lee HW, Lee KT, Kim CE (2000) Solid State Ionics 133:67
Jørgensen MJ, Primdahl S, Bagger C, Mogensen M (2001) Solid State Ionics 139:1
Murray E, Tsai T, Barnett S (2002) Solid State Ionics 110:235
Murray EP, Sever MJ, Barnett SA (2002) Solid State Ionics 148:27
Wang S, Kato T, Nagata S, Honda T, Kaneko T, Iwashita N, Dokiya M (2002) Solid State Ionics 146:203
Liu Y, Rauch W, Zha S, Liu M (2004) Solid State Ionics 166:261
Sarantaridis D, Atkinson A (2007) Fuel Cells 7:246
Iida T, Kawano M, Matsui T, Kikuchi R, Eguchi K (2007) J Electrochem Soc 154:B234
Li S, Wang S, Nie H, Wen TL (2007) J Solid State Electrochem 11:59
Jung GB, Lo KF, Chan SH (2007) J Solid State Electrochem 11:1435
van Berkel FPF, van Heuveln FH, Huijsmans JPP (1994) Solid State Ionics 72:240
Lee DS, Lee JH, Kim J, Lee HW, Song HS (2004) Solid State Ionics 166:13
Fukui T, Murata K, Ohara S, Abe H, Naito M, Nogi K (2004) J Power Sources 125:17
Schneider LCR, Martin CL, Bultel Y, Dessemond L, Bouvard D (2007) Electrochim Acta 52:3190
Kim SD, Moon H, Hyun SH, Moon J, Kim J, Lee HW (2007) Solid State Ionics 178:1304
Kong J, Sun K, Zhou D, Zhang N, Mu J, Qiao J (2007) J Power Sources 166:337
Sunde S (2000) J Electroceram 5:153
Sunde S (1997) Electrochim Acta 42:2637
Barfod R, Mogensen M, Klemensø T, Hagen A, Liu YL, Hendriksen PV (2007) J Electrochem Soc 154:B371
Sunde S (1996) J Electrochem Soc 143:1123
Aruna ST, Muthuraman M, Patil KC (1998) Solid State Ionics 111:45
Skarmoutsos D, Tsoga A, Naoumidis A, Nikolopoulos P (2000) Solid State Ionics 135:439
Anselmi-Tamburini U, Chiodelli G, Arimondi M, Maglia F, Spinolo G, Munir ZA (1998) Solid State Ionics 110:35
Corbin SF, Qiao X (2003) J Am Ceram Soc 86:401
Koide H, Someya Y, Yoshida T, Maruyama T (2000) Solid State Ionics 132:253
Simwonis D, Tietz F, Stöver D (2000) Solid State Ionics 132:241
Iwata T (1996) J Electrochem Soc 143:1521
Fouquet D, Müller AC, Weber A, Ivers-Tiffée E (2003) Ionics 8:103
Kim H, Liu C, Worrell WL, Vohs JM, Gorte RJ (2002) J Electrochem Soc 149:A247
Gorte RJ, Park S, Vohs JM, Wang C (2000) Adv Mater 12:1465
Sato K, Sakamaki K, Inoue Y (2000) Mater Trans JIM 41:1621
Laguna-Bercero MA, Larrea A, Penã JI, Merino RI, Orera VM (2005) J Eur Ceram Soc 25:1455
Tsipis EV, Kharton VV, Frade JR (2005) J Eur Ceram Soc 25:2623
Grgicak CM, Green RG, Giorgi JB (2006) J Mater Chem 16:885
Ishihara T, Shinagawa M, Kawakami A, Matsumoto H (2007) Mater Sci Forum 539–543:1350
Grgicak CM, Green RG, Giorgi JB (2008) J Power Sources 179:317
Ruiz-Morales JC, Nunez P, Buchanan R, Irvine JTS (2003) J Electrochem Soc 150:A1030
Huang P, Horky A, Petric A (1999) J Am Ceram Soc 82:2402
Huang K, Wan J-H, Goodenough JB (2001) J Electrochem Soc 148:A788
Kharton VV, Yaremchenko AA, Viskup AP, Mather GC, Naumovich EN, Marques F (2001) J Electroceram 7:57
Yokokawa H, Sakai N, Horita T, Yamaji K, Brito ME (2005) Electrochem 73:20
Naumovich EN, Kharton VV, Yaremchenko AA, Patrakeev MV, Kellerman DG, Logvinovich DI, Kozhevnikov VL (2006) Phys Rev B 74:064105
Brisse A, Sauvet A-L, Barthet C, Beaudet-Savignat S, Fouletier J (2006) Fuel Cells 6:59
Tsipis EV, Kharton VV, Frade JR (2007) Electrochim Acta 52:4428
Mori M, Hiei Y, Itoh H, Tompsett GA, Sammes NM (2003) Solid State Ionics 160:1
Skarmoutsos D, Tsoga A, Naoumidis A, Nikolopoulos P (2000) Solid State Ionics 135:439
Fagg DP, Feighery AJ, Irvine JTS (2003) J Solid State Chem 172:277
Mantzouris X, Zouvelou N, Haanappel VAC, Tietz F, Nikolopoulos P (2007) J Mater Sci 42:10152
Möbius H-H, Rohland B (1868) US Patent 3377203
Steele BCH (1999) Nature 400:619
Marina OA, Bagger C, Primdahl S, Mogensen M (1999) Solid State Ionics 123:199
Kharton VV, Naumovich EN, Tikhonovich VN, Bashmakov IA, Boginsky LS, Kovalevsky AV (1999) J Power Sources 79:242
Jiang SP, Duan YY, Love JG (2002) J Electrochem Soc 149:A1175
Tu H, Apfel H, Stimming U (2006) Fuel Cells 6:303
Kurokawa H, Sholklapper TZ, Jacobson CP, De Jonghe LC, Visco SJ (2007) Electrochem Solid State Lett 10:B135
Beresnev SM, Kuzin BL, Bronin DI (2007) Russ J Electrochem 43:883
Uchida H, Suzuki H, Watanabe M (1998) J Electrochem Soc 145:615
Ishihara T, Shibayama T, Nishiguchi H, Takita Y (2000) Solid State Ionics 132:209
Tsipis EV, Kharton VV, Bashmakov IA, Naumovich EN, Frade JR (2004) J Solid State Electrochem 8:674
Tsipis EV, Shlyakhtina AV, Shcherbakova LG, Kolbanev IV, Kharton VV, Vyshatko NP, Frade JR (2003) J Electroceram 10:153
Tsipis EV, Kharton VV, Vyshatko NP, Shaula AL, Frade JR (2003) J Solid State Chem 176:47
Kharton VV, Tsipis EV, Yaremchenko AA, Vyshatko NP, Shaula AL, Naumovich EN, Frade JR (2003) J Solid State Electrochem 7:468
Hornés A, Gamarra D, Munuera G, Conesa JC, Martínez-Arias A (2007) J Power Sources 169:9
Atkinson A, Barnett S, Gorte RJ, Irvine JTS, McEvoy AJ, Mogensen M, Singhal SC, Vohs J (2004) Nature Mater 3:17
Goodenough JB, Huang Y-H (2007) J Power Sources 173:1
Hui S, Petric A (2001) Solid State Ionics 143:275
Bazuev GV, Shveikin GP (1985) Complex oxides of elements with completing d- and f-shells. Nauka, Moscow
Vernoux P, Guindet J, Gehain E, Kleitz M (1997) Catalysts for continuous methane reforming in medium temperature SOFC. In: Stimming, U, Singhal, SC, Tagawa, H, Lehnert, W (eds) SOFC V. The Electrochemical Society, Pennington, NJ, pp 219–227
Vernoux P (1997) Ionics 3:270
Sfeir J, Buffat PA, Möckli P, Xanthopoulos N, Vasquez R, Mathieu HJ, Van herle J, Thampi KR (2001) J Catal 202:229
Vashook V, Vasylechko L, Zosel J, Müller R, Ahlborn E, Guth U (2004) Solid State Ionics 175:151
Georges S, Parrour G, Henault M, Fouletier J (2006) Solid State Ionics 177:2109
Baker RT, Metcalfe IS, Middleton PH, Steele BCH (1994) Solid State Ionics 72:328
Sauvet A-L, Guindet J, Fouletier J (1999) Ionics 5:150
Primdahl S, Hansen JR, Grahl-Madsen L, Larsen PH (2001) J Electrochem Soc 148:A74
Pudmich G, Boukamp BA, Gonzalez-Cuenca M, Jungen W, Zipprich W, Tietz F (2000) Solid State Ionics 135:433
Nabae Y, Yamanaka I, Takenaka S, Hatano M, Otsuka K (2005) Chem Lett 34:774
Madsen BD, Barnett SA (2007) J Electrochem Soc 154:B501
Sfeir J (2003) J Power Sources 118:276
Tietz F (1999) Ionics 5:129
Tao S, Irvine JTS (2004) J Electrochem Soc 151:A252
Liu J, Madsen B, Ji Z, Barnett S (2002) Electrochem Solid State Lett 5:A122
Huang Y-H, Dass RI, Denyszyn JC, Goodenough JB (2006) J Electrochem Soc 153:A1266
Kharton VV, Tsipis EV, Marozau IP, Viskup AP, Frade JR, Irvine JTS (2007) Solid State Ionics 178:101
Zha S, Tsang P, Cheng Z, Liu M (2005) J Solid State Chem 178:1844
Tao S, Irvine JTS, Kilner JA (2005) Adv Mater 17:1734
Peña-Martínez J, Marrero-López D, Ruiz-Morales JC, Savaniu C, Núñez P, Irvine JTS (2006) Chem Mater 18:1001
Jiang SP, Chen XJ, Chan SH, Kwok JT, Khor KA (2006) Solid State Ionics 177:149
Wan J, Zhu JH, Goodenough JB (2006) Solid State Ionics 177:1211
Chen XJ, Liu QL, Chan SH, Brandon NP, Khor KA (2007) J Electrochem Soc 154:B1206
Ruiz-Morales JC, Canalez-Vazquez J, Marrero-López D, Irvine JTS, Núñez P (2007) Electrochim Acta 52:7217
Sasaki K, Wurtz J-P, Gschwend R, Gödickemeier M, Gauckler LJ (1996) J Electrochem Soc 143:530
Bernuy-Lopez C, Allix M, Bridges CA, Claridge JB, Rosseinsky MJ (2007) Chem Mater 19:1035
Glöckner R, Neiman A, Larring Y, Norby T (1999) Solid State Ionics 125:369
Animitsa IE, Neiman AYA, Kochetova NA, Korona DV (2006) Russ J Electrochem 42:311
Zha S, Cheng Z, Liu M (2005) Electrochem Solid State Lett 8:A406
Jiang SP, Chan SH (2004) J Mater Sci 39:4405
Hui S, Petric A (2002) J Eur Ceram Soc 22:1673
Slater PR, Irvine JTS (1999) Solid State Ionics 124:61
Schouler EJL (1983) Solid State Ionics 9–10:945
Bohac P, Orliukas A, Gauckler L (1994) Lowering of the cathode overpotential of SOFC by electrolyte doping. In: Waser, R, Hoffmann, S, Bonnenberg, D, Hoffmann, Ch (eds) Electroceramics IV. vol. II. IWE, University of Technology, Augustinus Buchhandlung, Aachen, pp 771–780
van Hassel BA, Boukamp BA, Burggraaf AJ (1992) Solid State Ionics 51:161
Kurumchin EKh, Perfilyev MV (1990) Solid State Ionics 42:129
Juhl M, Primdahl S, Manon C, Mogensen M (1996) J Power Sources 61:173
Bae JM, Steele BCH (1998) Solid State Ionics 106:247
Kurumchin EKh (1998) Ionics 4:390
McEvoy AJ (2000) Solid State Ionics 135:331
Tomita A, Hibino T, Sano M (2005) Electrochem Solid State Lett 8:A333
Rose L, Kesler O, Tang Z, Burgess A (2007) J Power Sources 167:340
Thampi KR, McEvoy AJ, Van herle J (1995) J Electrochem Soc 142:506
Scholten D, Burggraaf AJ (1985) Solid State Ionics 16:147
Asano K, Iwahara H (1997) J Electrochem Soc 144:3125
Kharton VV, Viskup AP, Figueiredo FM, Naumovich EN, Shaulo AL, Marques FMB (2002) Mater Lett 53:160
de Ridder M, van Welzenis RG, Brongersma HH, Kreissig U (2003) Solid State Ionics 158:67
Fagg DP, Kharton VV, Frade JR (2004) J Solid State Electrochem 8:618
Fabry P, Kleitz M (1974) J Electroanal Chem 57:165
Kharton VV, Yaremchenko AA, Naumovich EN, Marques FMB (2000) J Solid State Electrochem 4:243
Kharton VV, Shaula AL, Patrakeev MV, Waerenborgh JC, Rojas DP, Vyshatko NP, Tsipis EV, Yaremchenko AA, Marques FMB (2004) J Electrochem Soc 151:A1236
Watanabe M, Uchida H, Shibata M, Mochizuki N, Amikura K (1994) J Electrochem Soc 141:342
Simner SP, Anderson MD, Templeton JW, Stevenson JW (2007) J Power Sources 168:236
Lu ZG, Zhu JH (2007) Electrochem Solid State Lett 10:B179
Rutenbeck D, Haanappel VAC, Uhlenbruck S, Tietz F, Vinke IC, Stöver D (2003) Noble metals in SOFC cathodes: processing and electrochemical performance. In: Singhal, SC, Dokiya, M (eds) SOFC-VIII. The Electrochemical Society, Pennington, NJ, pp 615–623
Uhlenbruck S, Tietz F, Haanappel VAC, Sebold D, Buchkremer HP, Stöver D (2004) J Solid State Electrochem 8:923
Sholklapper TZ, Radmilovich V, Jacobson CP, Visco SJ, De Jonghe LC (2008) J Power Sources 175:206
Datta P, Majewski P, Aldinger F (2008) Mater Chem Phys 107:370
Wang F-Y, Cheng S, Wan B-Z (2008) Catal Commun 9:1595
Rossmeisl J, Bessler WG (2008) Solid State Ionics 178:1694
Primdahl S, Mogensen M (2002) Solid State Ionics 152:597
Glaspell G, Hassan HMA, Elzatahry A, Abdalsayed V, El-Shall MS (2008) Top Catal 47:22
Llorca J, Casanovas A, Domingues M, Casanova I, Angurell I, Seco M, Rossell O (2008) J Nanopart Res 10:537
Tikhonovich VN, Kharton VV, Naumovich EN (1997) Inorg Mater 33:602
Sasaki K, Gödickemeier M, Bohac P, Orliukas A, Gauckler LJ (1993) Microstructure design of mixed-conducting solid oxide fuel cell cathodes. In: Biedermann P, Krahl-Urban B (eds) Proc 5th IEA Workshop on SOFC Materials, Process Engineering and Electrochemistry. Forschungszentrum Jülich, Germany, pp 187–196
Christie GM, van Berkel FPF (1996) Solid State Ionics 83:17
Tuller HL (2000) Solid State Ionics 131:143
Liu Y, Zha S, Liu M (2004) Chem Mater 16:3502
Aruna ST, Muthuraman M, Patil KC (1999) Solid State Ionics 120:275
Tikhonova LA (1991) PhD Thesis, Belarus State University, Minsk
Srilomsak S, Schilling D, Anderson HU (1989) Thermal expansion studies on cathode and interconnect oxides. In: Singhal, SC (eds) SOFC I. The Electrochemical Society, Pennington, NJ, pp 129–140
Tai LW, Nasrallah MM, Anderson HU, Sparlin DM, Sehlin SR (1995) Solid State Ionics 76:273
Tai LW, Nasrallah MM, Anderson HU, Sparlin DM, Sehlin SR (1995) Solid State Ionics 76:259
Yamamoto O, Takeda Y, Kanno R, Kojima T (1989) Stability of perovskite oxide electrode with stabilized zirconia. In: Singhal, SC (eds) SOFC I. The Electrochemical Society, Pennington, NJ, pp 242–253
Dees DW, Claar TD, Easler TE, Fee DC, Mrazek FC (1987) J Electrochem Soc 134:2141
Chiba R, Yoshimura F, Sakurai Y (2002) Solid State Ionics 152–153:575
Yu HC, Zhao F, Virkar AV, Fung KZ (2005) J Power Sources 152:22
Jiang SP, Ramprakash Y (1999) Solid State Ionics 116:145
Holtappels P, de Haart LGJ, Stimming U (1999) J Electrochem Soc 146:1620
Primdahl S, Mogensen M (1997) J Electrochem Soc 144:3409
Zhang X, Ohara S, Maric R, Mukai K, Fukui T, Yoshida H, Nishimura M, Inagaki T, Miura K (1999) J Power Sources 83:170
Maric R, Ohara S, Fukui T, Inagaki T, Fujita J (1998) Electrochem Solid State Lett 1:201
Petrov AN, Cherepanov VA, Zuev AYu (1987) Z Fiz Khim 61:630
Katsura T, Kitayama K, Sugihara T, Kimizuka N (1975) Bull Chem Soc Jpn 48:1809
Rice DE, Buttrey DJ (1993) J Solid State Chem 105:197
Kanai H, Mizusaki J, Tagawa H, Hoshiyama S, Hirano K, Fujita K, Tezuka M, Hashimoto T (1997) J Solid State Chem 131:150
Atsumi T, Ohgushi T, Kamegashira N (1996) J Alloys Compd 238:35
Tsipis EV, Naumovich EN, Shaula AL, Patrakeev MV, Waerenborgh JC, Kharton VV (2008) Solid State Ionics 179:57
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tsipis, E.V., Kharton, V.V. Electrode materials and reaction mechanisms in solid oxide fuel cells: a brief review. J Solid State Electrochem 12, 1367–1391 (2008). https://doi.org/10.1007/s10008-008-0611-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-008-0611-6