Abstract
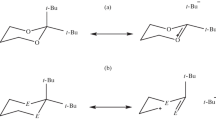
Complete basis set CBS-4, hybrid-density functional theory (hybrid-DFT: B3LYP/6-311+G**) based methods and natural bond orbital (NBO) interpretations have been used to examine the contributions of the hyperconjugative, electrostatic, and steric effects on the conformational behaviors of trans-2,3-dihalo-1,4-diselenane [halo = F (1), Cl (2), Br (3)] and trans-2,5-dihalo-1,4-diselenane [halo = F (4), Cl (5), Br (6)]. Both levels of theory showed that the axial conformation stability, compared to its corresponding equatorial conformation, decreases from compounds 1 → 3 and 4 → 6. Based on the results obtained from the NBO analysis, there are significant anomeric effects for compounds 1-6. The anomeric effect associated with the electron delocalization is in favor of the axial conformation and increases from compounds 1 → 3 and 4 → 6. On the other hand, dipole moment differences between the axial and equatorial conformations [Δ(μ eq - μ ax)] decrease from compounds 1 → 3. Although Δ(μ eq-μ ax) parameter decreases from compound 1 to compound 3, the dipole moment values of the axial conformations are smaller than those of their corresponding equatorial conformations. Therefore, the anomeric effect associated with the electron delocalizations (for halogen-C-Se segments) and the electrostatic model associated with the dipole-dipole interactions fail to account for the increase of the equatorial conformations stability on going from compound 1 to compound 3. Since there is no dipole moment for the axial and equatorial conformations of compounds 4-6, consequently, the conformational preferences in compounds 1-6 is in general dictated by the steric hindrance factor associated with the 1,3-syn-axial repulsions. Importantly, the CBS-4 results show that the entropy difference (∆S) between the equatorial axial conformations increases from compounds 1 → 3 and 4 → 6. This fact can be explained by the anomeric effect associated with the electron delocalization which affects the C2-Se bond orders and increase the rigidity of the corresponding rings. The Gibbs free energy difference values between the axial and equatorial conformations (i.e. ΔG ax-ax and ΔG eq-eq) of compounds 1 and 4, 2 and 5 and also 3 and 6 have been calculated. The correlations between the anomeric effect, electrostatic model, ΔG eq-ax, ΔG ax-ax, ΔG eq-eq, bond orders, dipole-dipole interactions, structural parameters and conformational behaviors of compounds 1-6 have been investigated.






Similar content being viewed by others
References
Bersuker IB (2006) The Jahn-Teller Effect. Cambridge University Press, Cambridge
Pozharskii AF, Soldatenkov AT, Katritzky AR (1997) Heterocycles in life and society. Wiley, New York
Valverde MG, Torroba T (2005) Sulfur-nitrogen heterocycles. Molecules 10:318–320. doi:10.3390/10020318
Liu RS (2001) Synthesis of oxygen heterocycles via alkynyltungsten compounds. Pure Appl Chem 73:265–269. doi:10.1351/pac200173020265
Reddy GPV, Kiran YB, Reddy SC, Reddy DC (2004) Synthesis and antimicrobial activity of novel phosphorus heterocycles with exocyclic P-C link. Chem Pharm Bull 52:307–310
Hafez A (2008) Selenium containing heterocycles: synthesis, anti-inflammatory, analgesic and anti-microbial activities of some new 4-cyanopyridazine-3(2H) selenone derivatives. Eur J Med Chem 43:1971–1977. doi:10.1016/j.ejmech.2007.12.006
Młochowski J (2008) Developments in the chemistry of selenaheterocyclic compounds of practical importance. Phosphorus Sulfur Silicon Relat Elem 183(4):931–938. doi:10.1080/10426500801898408
Epiotis ND, Yates RL, Larson RJ, Kirmayer CR, Bernardi F (1977) Directional effects of sigma. Conjugation on geometrical isomerism. J Am Chem Soc 99:8379–8388. doi:10.1021/ja00468a001
Dionne P, St-Jacques M (1987) Mechanism of the gauche conformational effect in 3-halogenated 1,5-benzodioxepins. J Am Chem Soc 109:2616–2623. doi:10.1021/ja00243a012
Eliel EL, Wilen SH (1994) Stereochemistry of organic compounds. Wiley, New York
Juaristi E, Cuevas G (1995) The anomeric effect. CRC, Boca Raton
Deslongchamps P (1983) Stereoelectronic effects in organic chemistry. Wiley, New York
Kirby J (1983) The anomeric effect and related stereoelectronic effects at oxygen. Springer, New York
Cramer CJ (1992) Anomeric and reverse anomeric effects in the gas phase and aqueous solution. J Org Chem 57:7034–7043. doi:10.1021/jo00052a012
Cramer CJ, Truhlar DG, French AD (1997) Exo-anomeric effects on energies and geometries of different conformations of glucose and related systems in the gas phase and aqueous solution. Carbohydr Res 298:1–14
Perrin CL, Armstrong KB, Fabian MA (1994) The origin of the anomeric effect: conformational analysis of 2-methoxy-1,3-dimethylhexahydropyrimidine. J Am Chem Soc 116:715–722. doi:10.1021/ja00081a037
Juaristi E, Cuevas G (1992) Recent studies of the anomeric effect. Tetrahedron 48:5019–5087. doi:10.1016/S0040-4020(01)90118-8
Lesarri A, Vega-Toribio A, Suenram RD, Brugh DJ, Nori-Shargh D, Boggs JE, Grabow J-U (2011) Structural evidence of anomeric effects in the anesthetic isoflurane. Phys Chem Chem Phys 13:6610–6618. doi:10.1039/c0cp02465a
Nori-Shargh D, Yahyaei H (2009) Stereoelectronic interaction effects (associated with the anomeric effects) on the conformational properties of 2-methylaminotetrahydropyran, 2-methylaminotetrahydrothiopyran, 2-methylaminotetrahydroselenopyran and their analogs containing P and As atoms: an ab initio study and NBO analysis. J Mol Struct (THEOCHEM) 913:8–15. doi:10.1016/j.theochem.2009.06.047
Nori-Shargh D, Yahyaei H, Mousavi SN, Kianpour M (2011) Conformational behaviors of 1,7-dioxa-spiro[5,5]undecane and its dithia and diselena analogs in relation to the anomeric effect: a hybrid-DFT, ab initio MO study and NBO interpretation. Comput Theor Chem 974:79–85. doi:10.1016/j.comptc.2011.07.014
Vila A, Mosquera RA (2007) Atoms in molecules interpretation of the anomeric effect in the OZCZO unit. J Comput Chem 28:1516–1530. doi:10.1002/jcc.20585
Altona C, Romers C, Havinga E (1959) Molecular structure and conformation of some dihalogenodioxanes. Tetrahedron Lett 1:16–20
Thacher GRJ (Ed.) (1993) The anomeric effect and associated steroelectronic effects. American Chemical Society, ACS Symposium Series No. 539, Washington DC
Alabugin IV (2000) Stereoelectronic interactions in cyclohexane, 1,3-dioxane, 1,3-oxathiane, and 1,3-dithiane: W-Effect, σC-X↔σ*C-H interactions, anomeric effect-what Is really important? J Org Chem 65:3910–3919. doi:10.1021/jo991622+
Nori-Shargh D, Hassanzadeh N, Kosari M, Rabieikarahroudi P, Yahyaei H, Sharifi S (2010) Stereoelectronic interaction effects on the conformational properties of 5-methyl-5-aza-1,3-dithiacyclohexane and its analogous containing N, P, O, and Se atoms-A hybrid density functional theory (DFT), ab initio study, and natural bond orbital (NBO) analysis. Can J Chem 88:579–587. doi:10.1139/V10-022
Azarakhshi F, Nori-Shargh D, Attar H, Masnabadi N, Yahyaei H, Mousavi SN, Boggs JE (2011) Conformational behaviors of 2-substituted cyclohexanones. A complete basis set, hybrid-DFT study and NBO interpretation. Mol Simul 37:1207–1220. doi:10.1080/08927022.2011.590986
Praly J-P, Lemieux RU (1987) Influence of solvent on the magnitude of the anomeric effect. Can J Chem 65:213–223. doi:10.1139/v87-034
Nori-Shargh D, Boggs JE (2011) Complete basis set, hybrid-DFT study and NBO interpretations of conformational behaviors of trans-2,3- and trans-2,5-dihalo-1,4-dithianes. J Phys Org Chem 24:212–221. doi:10.1002/poc.1728
Schmidt MW, Baldridge KK, Boatz JA, Elbert ST, Gordon MS, Jensen JH, Koseki S, Matsunaga N, Nguyen KA, Su SJ, Windus TL, Dupuis M, Montgomery JA (1993) General atomic and molecular electronic structure system. J Comput Chem 14:1347–1363. doi:10.1002/jcc.540141112
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Zakrzewski VG, Montgomery JA, Stratmann RE Jr, Burant JC, Dapprich S, Millam JM, Daniels AD, Kudin KN, Strain MC, Farkas O, Tomasi J, Barone V, Cossi M, Cammi R, Mennucci B, Pomelli C, Adamo C, Clifford S, Ochterski J, Petersson GA, Ayala PY, Cui Q, Morokuma K, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Cioslowski J, Ortiz JV, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Gomperts R, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Gonzalez C, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Andres JL, Head-Gordon M, Replogle ES, Pople JA (1998) GAUSSIAN 98 (Revision A.3). Gaussian Inc, Pittsburgh
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652. doi:10.1063/1.464913
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789. doi:10.1103/PhysRevB.37.785
Hehre WJ, Radom L, Schleyer PR, Pople JA (1986) Ab initio molecular orbital theory. Wiley, New York
Johnson BG, Seminario JM, Politzer P (eds) (1995) In modern density function theory: a tool for chemistry. Elsevier, Amsterdam
Glendening ED, Badenhoop JK, Reed AE, Carpenter JE, Bohmann JA, Morales CM, Weinhold F (2004) NBO Version 5.G. Theoretical Chemistry Institute. University of Wisconsin, Madison
Nyden MR, Petersson GA (1981) Complete basis set correlation energies. I. The asymptotic convergence of pair natural orbital expansions. J Chem Phys 75:1843–1862. doi:10.1063/1.442208
Petersson GA, Al-Laham MA (1991) A complete basis set model chemistry. II. Open-shell systems and the total energies of the first row atoms. J Chem Phys 94:6081–6090. doi:10.1063/1.460447
Petersson GA, Tensfeldt TG, Montgomery JA Jr (1991) A complete basis set model chemistry. III. The complete basis setquadratic configuration interaction family of methods. J Chem Phys 94:6091–6101. doi:10.1063/1.460448
Ochterski JW, Petersson GA, Montgomery JA Jr (1996) A complete basis set model chemistry. V. Extensions to six or more heavy atoms. J Chem Phys 104:2598–2619. doi:10.1063/1.470985
Petersson GA, Malick DK, Wilson WG, Ochterski JW, Montgomery JA Jr (1998) Calibration and comparison of the Gaussian- 2, complete basis set, and density functional methods for computational thermochemistry. J Chem Phys 109:10570–10579. doi:10.1063/1.477794
Montgomery JA Jr, Frisch MJ, Ochterski JW, Petersson GA (1999) A complete basis set model chemistry. VI. Use of density functional geometries and frequencies. J Chem Phys 110:2822–2827. doi:10.1063/1.477924
Montgomery JA Jr, Frisch MJ, Ochterski JW, Petersson GA (2000) A complete basis set model chemistry. VII. Use of the minimum population localization method. J Chem Phys 112:6532–6542. doi:10.1063/1.481224
Weinhold F (2003) Rebuttal to the bickelhaupt–baerends case for steric repulsion causing the staggered conformation of ethane. Angew Chem Int Ed 42:4188–4194. doi:10.1002/anie.200351777
Wiberg KB, Murcko MA (1987) Rotational barriers. 1. 1,2- Dihaloethanes. J Phys Chem 91:3616–3620. doi:10.1021/j100297a030
Mo Y (2010) Computational evidence that hyperconjugative interactions are not responsible for the anomeric effect. Nat Chem 2:666–671. doi:10.1038/nchem.721
Liu SB (2007) Steric effect: A quantitative description from density functional theory. J Chem Phys 126(1-5):244103. doi:10.1063/1.2747247
Huang Y, Zhong A-G, Yang Q, Liu SB (2011) Origin of anomeric effect: a density functional steric analysis. J Chem Phys 134(1-9):084103. doi:10.1063/1.3555760
Acknowledgments
We thank Dr. Daryoush Tahmasebi for CBS-4 calculations.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Dedicated to the memory of Professor James E. Boggs
Rights and permissions
About this article
Cite this article
Nori-Shargh, D., Mousavi, S.N. & Kayi, H. Conformational behaviors of trans-2,3- and trans-2,5-dihalo-1,4-diselenanes. A complete basis set, hybrid-density functional theory study and natural bond orbital interpretations. J Mol Model 20, 2249 (2014). https://doi.org/10.1007/s00894-014-2249-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-014-2249-x