Abstract
A T-DNA tagged mutant line of Arabidopsis thaliana, produced with a promoter trap vector carrying a promoterless gus (uidA) as a reporter gene, showed GUS induction in response to mechanical wounding. Cloning of the chromosomal DNA flanking the T-DNA revealed that the insert had caused a knockout mutation in a PTR-type peptide transporter gene named At5g46050 in GenBank, here renamed AtPTR3. The gene and the deduced protein were characterized by molecular modelling and bioinformatics. Molecular modelling of the protein with fold recognition identified 12 transmembrane spanning regions and a large loop between the sixth and seventh helices. The structure of AtPTR3 resembled the other PTR-type transporters of plants and transporters in the major facilitator superfamily. Computer analysis of the AtPTR3 promoter suggested its expression in roots, leaves and seeds, complex hormonal regulation and induction by abiotic and biotic stresses. The computer-based hypotheses were tested experimentally by exposing the mutant plants to amino acids and several stress treatments. The AtPTR3 gene was induced by the amino acids histidine, leucine and phenylalanine in cotyledons and lower leaves, whereas a strong induction was obtained in the whole plant upon exposure to salt. Furthermore, the germination frequency of the mutant line was reduced on salt-containing media, suggesting that the AtPTR3 protein is involved in stress tolerance in seeds during germination.
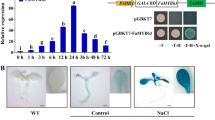
Figure a Induction of AtPTR3 gene by amino acids. GUS staining of line 9 plants eight hours after induction with amino acids. Control indicates plant treated with water. His, Leu and Phe indicate plants treated with 10 mM amino acids histidine, leucine or phenylalanine, respectively. b Induction of AtPTR3 gene by salt. GUS staining of line 9 plants grown on MS medium on different salt concentrations: Control indicates plant grown on MS medium and 100 mM, 120 mM and 140 mM indicate plants grown on MS medium supplemented with the indicated NaCl concentrations. Size of the plants grown on salt medium has been magnified. c Germination frequency of Atptr3 knockout mutant line is reduced on salt medium. Atptr3 knockout mutant (9) and wild type C24 (WT) sown on MS medium (Control) and MS medium supplemented with salt (140 mM NaCl).







Similar content being viewed by others
References
Bowie JU, Luethy R, Eisenberg D (1991) Science 253:164–170
Jones DT (1999) J Mol Biol 287:797–815
Jones DT (2000) Curr Opin Struct Biol 10:371–379
Sánchez R, Pieper U, Melo F, Eswar N, Martí-Renom MA, Madhusudhan MS, Mirkovic N, Šali A (2000) Nat Struct Biol 7:986–990
Brenner SE, Chothia C, Hubbard TJP (1997) Curr Opin Struct Biol 7:369–376
Šali A, Matsumoto R, McNeil HP, Karplus M, Stevens RL (1993) J Biol Chem 268:9023–9034
Fetrow JS, Godzik A, Skolnick J (1998) J Mol Biol 282:703–711
Svensson M, Lundh D, Ejdebäck M, Mandal A (2004) J Mol Model 10:130–138
Martí-Renom MA, Stuart AC, Fiser A, Sánchez R, Melo F, Šali A (2000) Annu Rev Biophys Biomol Struct 29:291–325
Arabidopsis Genome Initiative (2000) Nature 408:796–815
Springer PS (2000) Plant Cell 12:1007–1020
Valentine L (2003) Plant Physiol 133:948–955
Lindsey K, Wei W, Clarke MC, McArdle HF, Rooke LM, Topping JF (1993) Transgenic Res 2:33–47
Mathur J, Szabados L, Schaefer S, Grunenberg B, Lossow A, Jonas-Straube E, Schell J, Koncz C, Koncz-Kalman Z (1998) Plant J 13:707–716
Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, Gadrinab C, Heller C, Jeske A, Koesema E, Meyers CC, Parker H, Prednis L, Ansari Y, Choy N, Deen H, Geralt M, Hazari N, Hom E, Karnes M, Mulholland C, Ndubaku R, Schmidt I, Guzman P, Aguilar-Henonin L, Schmid M, Weigel D, Carter DE, Marchand T, Risseeuw E, Brogden D, Zeko A, Crosby WL, Berry CC, Ecker JR (2003) Science 301:653–657
Pao SS, Paulsen IT, Saier MH Jr (1998) Microbiol Mol Biol Rev 62:1–34
Abramson J, Iwata S, Kaback HR (2004) Mol Membr Biol 21:227–236
Abramson J, Smirnova I, Kasho V, Verner G, Kaback HR, Iwata S (2003) Science 301:610–615
Huang Y, Lemieux MJ, Song J, Auer M, Wang D-N (2003) Science 301:616–620
Hirai T, Heymann JAW, Maloney PC, Subramaniam S (2003) J Bacteriol 185:1712–1718
Vardy E, Arkin IT, Gottschalk KE, Kaback HR, Schuldiner S (2004) Protein Sci 13:1832–1840
Saier MH Jr (2000) Microbiol Mol Biol Rev 64:354–411
Saier MH Jr (2000) Microbiology 146:1775–1795
Paulsen IT, Skurray RA (1994) Trends Biochem Sci 19:404–405
Steiner HY, Naider F, Becker JM (1995) Mol Microbiol 16:825–834
Chang AB, Lin R, Studley WK, Tran CV, Saier MH Jr (2004) Mol Membr Biol 21:171–181
Chiang C-S, Stacey G, Tsay Y-F (2004) J Biol Chem 279:30150–30157
Huang N-C, Liu K-H, Lo H-J, Tsay Y-F (1999) Plant Cell 11:1381–1392
Lin C-M, Koh S, Stacey G, Yu S-M, Lin T-Y, Tsay Y-F (2000) Plant Physiol 122:379–388
Galván A, Fernández E (2001) Cell Mol Life Sci 58:225–233
Frommer WB, Hummel S, Rentsch D (1994) FEBS Lett 347:185–189
Zhou J-J, Theodoulou FL, Muldin I, Ingemarsson B, Miller AJ (1998) J Biol Chem 273:12017–12023
Jeong J, Suh SJ, Guan C, Tsay Y-F, Moran N, Oh CJ, An CS, Demchenko KN, Pawlowski K, Lee Y (2004) Plant Physiol 134:969–978
Stacey G, Koh S, Granger C, Becker JM (2002) Trends Plant Sci 7:257–263
Song W, Steiner H-Y, Zhang L, Naider F, Stacey G, Becker JM (1996) Plant Physiol 110:171–178
Song W, Koh S, Czako M, Marton L, Drenkard E, Becker JM, Stacey G (1997) Plant Physiol 114:927–935
Rentsch D, Laloi M, Rouhara I, Schmelzer E, Delrot S, Frommer WB (1995) FEBS Lett 370:264–268
Dietrich D, Hammes U, Thor K, Suter-Grotemeyer M, Fluckiger R, Slusarenko AJ, Ward JM, Rentsch D (2004) Plant J 40:488–499
Tsay YF, Schroeder JI, Feldmann KA, Crawford NM (1993) Cell 72:705–713
Guo FQ, Wang R, Chen M, Crawford NM (2001) Plant Cell 13:1761–1777
Okamoto M, Vidmar JJ, Glass ADM (2003) Plant Cell Physiol 44:304–317
Mandal A, Sandgren M, Holmström K-O, Gallois P, Palva ET (1995) Plant Mol Biol Rep 13:243–254
Murashige T, Skoog F (1962) Phys Plantarum 15:473–497
Southern E (1975) J Mol Biol 98:503–517
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor NY
Benson DA, Karsch-Mizrachi I, Lipman DJ, Ostell J, Rapp BA, Wheeler DL (2000) Nucleic Acids Res 28:15–18
Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE (2000) Nucleic Acids Res 28:235–242
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) J Mol Biol 215:403–410
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Nucleic Acids Res 25:3389–3402
Apweiler R, Attwood TK, Bairoch A, Bateman A, Birney E, Biswas M, Bucher P, Cerutti L, Corpet F, Croning MDR, Durbin R, Falquet L, Fleischmann W, Gouzy J, Hermjakob H, Hulo N, Jonassen I, Kahn D, Kanapin A, Karavidopoulou Y, Lopez R, Marx B, Mulder NJ, Oinn TM, Pagni M, Servant F, Sigrist CJA, Zdobnov EM (2001) Nucleic Acids Res 29:37–40
Thompson JD, Higgins DG, Gibson TJ (1994) Nucleic Acids Res 22:4673–4680
Sonnhammer ELL, von Heijne G, Krogh A (1998) A hidden Markov model for predicting transmembrane helices in protein sequences. In: Glasgow J, Littlejohn T, Major F, Lathrop R, Sankoff D, Sensen C (eds) Proc ISMB. AAAI Press, Menlo Park, CA, pp175–182
Käll L, Krogh A, Sonnhammer ELL (2004) J Mol Biol 338:1027–1036
Jones DT, Taylor WR, Thornton JM (1994) Biochemistry 33:3038–3049
Jones DT (1998) FEBS Lett 423:281–285
von Heijne G (1992) J Mol Biol 225:487–494
Nakai K, Horton P (1999) Trends Biochem Sci 24:34–35
Kelley LA, MacCallum RM, Sternberg MJE (2000) J Mol Biol 299:499–520
Jones DT (1997) Curr Opin Struct Biol 7:377–387
Sánchez R, Šali A (1997) Curr Opin Struct Biol 7:206–214
Laskowski RA, MacArthur MW, Moss DS, Thornton JM (1993) J Appl Cryst 26:283–291
Meredith D, Boyd CAR (2000) Cell Mol Life Sci 57:754–778
Morris AL, MacArthur MW, Hutchinson EG, Thornton JM (1992) Proteins 12:345–364
Weinglass AB, Kaback HR (2000) Proc Natl Acad Sci USA 97:8938–8943
Seok Y-J, Sun J, Kaback HR, Peterkofsky A (1997) Proc Natl Acad Sci USA 94:13515–13519
Guo F-Q, Young J, Crawford NM (2003) Plant Cell 15:107–117
Cheong YH, Chang H-S, Gupta R, Wang X, Zhu T, Luan S (2002) Plant Physiol 129:661–677
Reymond P, Weber H, Damond M, Farmer EE (2000) Plant Cell 12:707–719
Denekamp M, Smeekens SC (2003) Plant Physiol 132:1415–1423
Waterworth WM, West CE, Bray CM (2000) J Exp Bot 51:1201–1209
Truernit E, Schmid J, Epple P, Illig J, Sauer N (1996) Plant Cell 8:2169–2182
Meyer S, Lauterbach C, Niedermeier M, Barth I, Sjolund RD, Sauer N (2004) Plant Physiol 134:684–693
Miranda M, Borisjuk L, Tewes A, Dietrich D, Rentsch D, Weber H, Wobus U (2003) Plant Physiol 132:1950–1960
Acknowledgements
The research grant from Nilsson-Ehle Foundation is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karim, S., Lundh, D., Holmström, KO. et al. Structural and functional characterization of AtPTR3, a stress-induced peptide transporter of Arabidopsis. J Mol Model 11, 226–236 (2005). https://doi.org/10.1007/s00894-005-0257-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-005-0257-6