Abstract
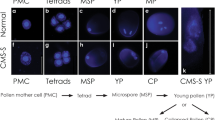
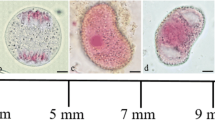
Like most angiosperms, wheat (Triticum aestivum) shows maternal inheritance of plastids. It is thought that this takes place by cytoplasmic stripping at fertilisation rather than the absence of plastids in sperm cells. To determine the fate of plastids during sperm cell development, plastid-targeted green fluorescent protein was used to visualise these organelles in nuclear transgenic wheat lines. Fewer than thirty small 1–2-μm plastids were visible in early uninucleate pollen cells. These dramatically increased to several hundred larger (4 μm) plastids during pollen maturation and went through distinct morphological changes. Only small plastids were visible in generative cells (n = 25) and young sperm cells (n = 9). In mature sperm cells, these green fluorescent protein (GFP)-tagged plastids were absent. This is consistent with maternal inheritance of plastids resulting from their degradation in mature sperm cells in wheat.



Similar content being viewed by others
References
Birky CW (1995) Uniparental inheritance of mitochondrial and chloroplast genes—mechanisms and evolution. Proc Natl Acad Sci U S A 92:11331–11338. doi:10.1073/pnas.92.25.11331
Bock R (2007) Structure, function and inheritance of plastid genomes. In: Cell and Molecular Biology of Plastids. Topics in Current Genetics, vol 19. Springer-Verlag, Berlin
Briggle LW (1966) Inheritance of a variegated leaf pattern in hexaploid wheat. Crop Sci 6:43–45
Chiba A, Ishida H, Nishizawa NK, Makino A, Mae T (2003) Exclusion of ribulose-1, 5-bisphosphate carboxylase/oxygenase from chloroplasts by specific bodies in naturally senescing leaves of wheat. Plant Cell Physiol 44:914–921. doi:10.1093/Pcp/Pcg118
Corriveau JL, Coleman AW (1988) Rapid screening method to detect potential biparental inheritance of plastid DNA and results for over 200 angiosperm species. Am J Bot 75:1443–1458. doi:10.2307/2444695
Cran DG, Possingham JV (1972) Variation of plastid types in spinach. Protoplasma 74:345–356. doi:10.1007/Bf01282537
Daniell H, Datta R, Varma S, Gray S, Lee SB (1998) Containment of herbicide resistance through genetic engineering of the chloroplast genome. Nat Biotechnol 16:345–348. doi:10.1038/Nbt0498-345
Day A, Ellis THN (1984) Chloroplast DNA deletions associated with wheat plants regenerated from pollen—possible basis for maternal inheritance of chloroplasts. Cell 39:359–368. doi:10.1016/0092-8674(84)90014-X
Dvorak J, Stokrova J (1993) Structure of the needles in the early phases of development in Pinus-Ponderosa Lawson, P. Et Lawson, C. with special reference to plastids. Ann Bot-London 72:423–431. doi:10.1006/anbo.1993.1128
Fujiwara MT, Hashimoto H, Kazama Y, Hirano T, Yoshioka Y, Aoki S, Sato N, Itoh RD, Abe T (2010) Dynamic morphologies of pollen plastids visualised by vegetative-specific FtsZ1-GFP in Arabidopsis thaliana. Protoplasma 242:19–33
Gardner IC, Abbas H, Scott A (1989) The occurrence of ameboid plastids in the actinorhizal root-nodules of Alnus-Glutinosa (L) Gaertn. Plant Cell Environ 12:205–211. doi:10.1111/j.1365-3040.1989.tb01934.x
Greiner S, Sobanski J, Bock R (2015) Why are most organelle genomes transmitted maternally? Bioessays 37:80–94. doi:10.1002/bies.201400110
Hagemann R, Schroder MB (1985) New results about the presence of plastids in generative and sperm cells of Gramineae. In: Sexual reproduction in seed plants, ferns and mosses. PUDOC, Wagenigen, pp 53–55
Hagemann R, Schroder MB (1989) The cytological basis of the plastid inheritance in angiosperms. Protoplasma 152:57–64. doi:10.1007/Bf01323062
Hu S, Zhu C, Xu L, J. S (1979) Ultrastructure of male gametophyte in wheat 1. The formation of generative and vegetative cells. Acta Botanica Sinica 21:208–214
Leech RM, Thomson WW, Platt-Aloia KA (1981) Observations on the mechanism of chloroplast division in higher plants. New Phytol 87:1–9. doi:10.1111/j.1469-8137.1981.tb01686.x
Lyndon RF, Robertson ES (1976) Quantitative ultrastructure of pea shoot apex in elation to leaf initiation. Protoplasma 87:387–402. doi:10.1007/Bf01624007
Matsushima R, Tang LY, Zhang L, Yamada H, Twell D, Sakamoto W (2011) A conserved, Mg2+-dependent exonuclease degrades organelle DNA during Arabidopsis pollen development. Plant Cell 23:1608–1624. doi:10.1105/tpc.111.084012
Michaeli S, Honig A, Levanony H, Peled-Zehavi H, Galili G (2014) Arabidopsis ATG8-INTERACTING PROTEIN1 is involved in autophagy-dependent vesicular trafficking of plastid proteins to the vacuole. Plant Cell 26:4084–4101. doi:10.1105/tpc.114.129999
Minamikawa T, Toyooka K, Okamoto T, Hara-Nishimura I, Nishimura M (2001) Degradation of ribulose-bisphosphate carboxylase by vacuolar enzymes of senescing French bean leaves: immunocytochemical and ultrastructural observations. Protoplasma 218:144–153. doi:10.1007/Bf01306604
Miyamura S, Kuroiwa T, Nagata T (1987) Disappearance of plastid and mitochondrial nucleoids during the formation of generative cells of higher plants revealed by fluorescence microscopy. Protoplasma 141:149–159. doi:10.1007/Bf01272897
Mogensen HL (1988) Exclusion of male mitochondria and plastids during syngamy in barley as a basis for maternal inheritance. Proc Natl Acad Sci U S A 85:2594–2597. doi:10.1073/pnas.85.8.2594
Mogensen HL (1996) The hows and whys of cytoplasmic inheritance in seed plants. Am J Bot 83:383–404. doi:10.2307/2446172
Mogensen HL, Rusche ML (1985) Quantitative ultrastructural analysis of barley sperm.1. Occurrence and mechanism of cytoplasm and organelle reduction and the question of sperm dimorphism. Protoplasma 128:1–13. doi:10.1007/BF01273229
Nagata N (2010) Mechanisms for independent cytoplasmic inheritance of mitochondria and plastids in angiosperms. J Plant Res 123:193–199. doi:10.1007/s10265-009-0293-x
Newcomb EH (1967) Fine structure of protein-storing plastids in bean root tips. J Cell Biol 33:143–163. doi:10.1083/Jcb.33.1.143
Osteryoung KW, Pyke KA (2014) Division and dynamic morphology of plastids. Annu Rev Plant Biol 65(65):443–472. doi:10.1146/annurev-arplant-050213-035748
Pao WK, Li HW (1946) Maternal inheritance of variegation in common wheat. J Am Soc Agron 38:90–94
Pierson ES (1988) Rhodamine-phalloidin staining of F-actin in pollen after dimethylsulfoxide permeabilization. Sex Plant Reprod 1:83–87
Primavesi LF, Wu H, Mudd EA, Day A, Jones HD (2008) Visualisation of plastids in endosperm, pollen and roots of transgenic wheat expressing modified GFP fused to transit peptides from wheat SSU RubisCO, rice FtsZ and maize ferredoxin III proteins. Transgenic Res 17:529–543. doi:10.1007/s11248-007-9126-7
Pyke KA (2010) Plastid division AoB PLANTS 2010:plq016-plq016 doi:10.1093/aobpla/plq016
Ruf S, Karcher D, Bock R (2007) Determining the transgene containment level provided by chloroplast transformation. Proc Natl Acad Sci U S A 104:6998–7002. doi:10.1073/pnas.0700008104
Schattat MH, Griffiths S, Mathur N, Barton K, Wozny MR, Dunn N, Greenwood JS, Mathur J (2012) Differential coloring reveals that plastids do not form networks for exchanging macromolecules. Plant Cell 24:1465–1477. doi:10.1105/tpc.111.095398
Schattat MH, Barton KA, Mathur J (2015) The myth of interconnected plastids and related phenomena. Protoplasma 252:359–371. doi:10.1007/s00709-014-0666-4
Schroder MB, Hagemann R (1986) Ultrastructural studies on plastids of generative and vegetative cells in Liliaceae.6. Patterns of plastid distribution during generative cell-formation in Aloe secundiflora and Aloe jucunda. Acta Bot Neerl 35:243–248
Sheppard AE, Ayliffe MA, Blatch L, Day A, Delaney SK, Khairul-Fahmy N, Li Y, Madesis P, Pryor AJ, Timmis JN (2008) Transfer of plastid DNA to the nucleus is elevated during male gametogenesis in tobacco. Plant Physiol 148:328–336. doi:10.1104/pp.108.119107
Svab Z, Maliga P (2007) Exceptional transmission of plastids and mitochondria from the transplastomic pollen parent and its impact on transgene containment. Proc Natl Acad Sci U S A 104:7003–7008. doi:10.1073/pnas.0700063104
Svab Z, Hajdukiewicz P, Maliga P (1990) Stable transformation of plastids in higher plants. Proc Natl Acad Sci U S A 87:8526–8530. doi:10.1073/pnas.87.21.8526
Wang SH, Blumwald E (2014) Stress-induced chloroplast degradation in Arabidopsis is regulated via a process independent of autophagy and senescence-associated Vacuoles. Plant Cell 26:4875–4888. doi:10.1105/tpc.114.133116
Wittenbach VA, Lin W, Hebert RR (1982) Vacuolar localization of proteases and degradation of chloroplasts in mesophyll protoplasts from senescing primary wheat leaves. Plant Physiol 69:98–102. doi:10.1104/Pp.69.1.98
Acknowledgments
Rothamsted Research receives support from the Biotechnological and Biological Sciences Research Council (BBSRC) of the UK as part of the 20:20 Wheat® Programme.
Work was supported by grant number GM114215. We thank Caroline Sparks for her invaluable help and also Richard Parkinson and Fiona Gilzean for their assistance in growing the wheat plants.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Benedikt Kost
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 1.62 mb)
Rights and permissions
About this article
Cite this article
Primavesi, L.F., Wu, H., Mudd, E.A. et al. Visualisation of plastid degradation in sperm cells of wheat pollen. Protoplasma 254, 229–237 (2017). https://doi.org/10.1007/s00709-015-0935-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-015-0935-x