Abstract
Background
Hepatocellular carcinoma (HCC) has a high mortality rate, and early detection of HCC improves patient survival. However, the molecular diagnostic markers for early HCC have not been fully elucidated. The aim of this study was to identify novel diagnostic markers for HCC.
Methods
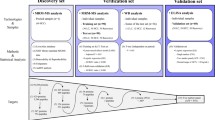
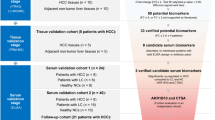
Serum protein profiles of 45 hepatitis C virus infection (HCV)-related HCC patients (HCV-HCC) were compared to 42 HCV-related chronic liver disease patients without HCC (HCV-CLD) and 21 healthy volunteers using the ProteinChip SELDI system. One of the identified proteins was evaluated as a diagnostic marker for HCC in patients with HCV.
Results
Five protein peaks (4067, 4470, 7564, 7929, and 8130 m/z) had p-values less than 1 × 10−7 and were significantly increased in the sera of HCV-HCC patients compared to HCV-CLD patients and healthy volunteers. Among these proteins, an 8130 m/z peak was the most differentially expressed and identified as the complement component 3a (C3a) fragment. For HCV-HCC and HCV-CLD, the relative intensity of this C3a fragment had the best area under the ROC curve [0.70], followed by des-γ-carboxy prothrombin (DCP) [0.68], lectin-bound alpha fetoprotein (AFP-L3) [0.58] and AFP [0.53] for HCC. A combined analysis of the C3a fragment, AFP and DCP led to a 98% positive identification rate. In addition, the measurable C3a fragment in some HCC patients was not only significantly higher in the year of HCC onset compared to the pre-onset year, but also decreased after treatment.
Conclusions
The 8130 m/z C3a fragment is a potential marker for the early detection of HCV-related HCC.




Similar content being viewed by others
References
El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745–50.
Robert GG. Hepatocellular carcinoma: overcoming challenges in disease management. Clin Gastroenterol Hepatol. 2006;4:252–61.
Okuda K. Hepatocellular carcinoma. J Hepatol. 2000;32:225–37.
Oka H, Tamori A, Kuroki T, Kobayashi K, Yamamoto S. Prospective study of alpha-fetoprotein in cirrhotic patients monitored for development of hepatocellular carcinoma. Hepatology. 1994;19:61–6.
Ishii M, Gama H, Chida N, Ueno Y, Shinzawa H, Takagi T, et al. Simultaneous measurements of serum alpha-fetoprotein and protein induced by vitamin K absence for detecting hepatocellular carcinoma. South Tohoku District Study Group. Am J Gastroenterol. 2000;95:1036–40.
Okuda H, Nakanishi T, Takatsu K, Saito A, Hayashi N, Takasaki K, et al. Serum levels of des-gamma-carboxy prothrombin measured using the revised enzyme immunoassay kit with increased sensitivity in relation to clinicopathologic features of solitary hepatocellular carcinoma. Cancer. 2000;88:544–9.
Grazi GL, Mazziotti A, Legnani C, Jovine E, Miniero R, Gallucci A, et al. The role of tumor markers in the diagnosis of hepatocellular carcinoma, with special reference to the des-gamma-carboxy prothrombin. Liver Transpl Surg. 1995;1:249–55.
Wang CS, Lin CL, Lee HC, Chen KY, Chiang MF, Chen HS, et al. Usefulness of serum des-gamma-carboxy prothrombin in detection of hepatocellular carcinoma. World J Gastroenterol. 2005;11:6115–9.
Marrero JA, Su GL, Wei W, Emick D, Conjeevaram HS, Fontana RJ, et al. Des-gamma carboxyprothrombin can differentiate hepatocellular carcinoma from nonmalignant chronic liver disease in American patients. Hepatology. 2003;37:1114–21.
Mita Y, Aoyagi Y, Yanagi M, Suda T, Suzuki Y, Asakura H. The usefulness of determining des-gamma-carboxy prothrombin by sensitive enzyme immunoassay in the early diagnosis of patients with hepatocellular carcinoma. Cancer. 1998;82:1643–8.
Taketa K, Okada S, Win N, Hlaing NK, Wind KM. Evaluation of tumor markers for the detection of hepatocellular carcinoma in Yangon General Hospital, Myanmar. Acta Med Okayama. 2002;56:317–20.
Khien VV, Mao HV, Chinh TT, Ha PT, Bang MH, Lac BV, et al. Clinical evaluation of lentil lectin-reactive alpha-fetoprotein-L3 in histology-proven hepatocellular carcinoma. Int J Biol Markers. 2001;16:105–11.
Zinkin NT, Grall F, Bhaskar K, Otu HH, Spentzos D, Kalmowitz B, et al. Serum proteomics and biomarkers in hepatocellular carcinoma and chronic liver disease. Clin Cancer Res. 2008;14:470–7.
Schwegler EE, Cazares L, Steel LF, Adam BL, Johnson DA, Semmes OJ, et al. SELDI-TOF MS profiling of serum for detection of the progression of chronic hepatitis C to hepatocellular carcinoma. Hepatology. 2005;41:634–42.
Kanmura S, Uto H, Kusumoto K, Ishida Y, Hasuike S, Nagata K, et al. Early diagnostic potential for hepatocellular carcinoma using the SELDI ProteinChip system. Hepatology. 2007;45:948–56.
Paradis V, Degos F, Dargère D, Pham N, Belghiti J, Degott C, et al. Identification of a new marker of hepatocellular carcinoma by serum protein profiling of patients with chronic liver diseases. Hepatology. 2005;41:40–7.
Lee IN, Chen CH, Sheu JC, Lee HS, Huang GT, Chen DS, et al. Identification of complement C3a as a candidate biomarker in human chronic hepatitis C and HCV-related hepatocellular carcinoma using a proteomics approach. Proteomics. 2006;6:2865–73.
Uto H, Hayashi K, Kusumoto K, Hasuike S, Nagata K, Kodama M, et al. Spontaneous elimination of hepatitis C virus RNA in individuals with persistent infection in a hyperendemic area of Japan. Hepatol Res. 2006;34:28–34.
Shevchenko A, Wilm M, Vorm O, Mann M. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal Chem. 1996;68:850–8.
Prahalad AK, Hickey RJ, Huang J, Hoelz DJ, Dobrolecki L, Murthy S, et al. Serum proteome profiles identifies parathyroid hormone physiologic response. Proteomics. 2006;6:3482–93.
Shiwa M, Nishimura Y, Wakatabe R, Fukawa A, Arikuni H, Ota H, et al. Rapid discovery and identification of a tissue-specific tumor biomarker from 39 human cancer cell lines using the SELDI ProteinChip platform. Biochem Biophys Res Commun. 2003;309:18–25.
Adam BL, Qu Y, Davis JW, Ward MD, Clements MA, Cazares LH, et al. Serum protein fingerprinting coupled with a pattern-matching algorithm distinguishes prostate cancer from benign prostate hyperplasia and healthy men. Cancer Res. 2002;62:3609–14.
Sahu A, Lambris JD. Structure and biology of complement protein C3, a connecting link between innate and acquired immunity. Immunol Rev. 2001;180:35–48.
Miguet L, Bogumil R, Decloquement P, Herbrecht R, Potier N, Mauvieux L, et al. Discovery and identification of potential biomarkers in a prospective study of chronic lymphoid malignancies using SELDI-TOF-MS. J Proteome Res. 2006;5:2258–69.
Ward DG, Suggett N, Cheng Y, Wei W, Johnson H, Billingham LJ, et al. Identification of serum biomarkers for colon cancer by proteomic analysis. Br J Cancer. 2006;94:1898–905.
Li J, Orlandi R, White CN, Rosenzweig J, Zhao J, Seregni E, et al. Independent validation of candidate breast cancer serum biomarkers identified by mass spectrometry. Clin Chem. 2005;51:2229–35.
Jurianz K, Ziegler S, Garcia-Schüler H, Kraus S, Bohana-Kashtan O, Fishelson Z, et al. Complement resistance of tumor cells: basal and induced mechanisms. Mol Immunol. 1999;36:929–39.
Bjørge L, Hakulinen J, Vintermyr OK, Jarva H, Jensen TS, Iversen OE, et al. Ascitic complement system in ovarian cancer. Br J Cancer. 2005;92:895–905.
Mollnes TE, Garred P, Bergseth G. Effect of time, temperature and anticoagulants on in vitro complement activation: consequences for collection and preservation of samples to be examined for complement activation. Clin Exp Immunol. 1988;73:484–8.
Verhaegen H, De Cock W, De Cree J, Verbruggen F. Increase of serum complement levels in cancer patients with progressing tumors. Cancer. 1976;38:1608–13.
Habermann JK, Roblick UJ, Luke BT, Prieto DA, Finlay WJ, Podust VN, et al. Increased serum levels of complement C3a anaphylatoxin indicate the presence of colorectal tumors. Gastroenterology. 2006;131:1020–9.
Steel LF, Shumpert D, Trotter M, Seeholzer SH, Evans AA, London WT, et al. A strategy for the comparative analysis of serum proteomes for the discovery of biomarkers for hepatocellular carcinoma. Proteomics. 2003;3:601–9.
Kawakami T, Hoshida Y, Kanai F, Tanaka Y, Tateishi K, Ikenoue T, et al. Proteomic analysis of sera from hepatocellular carcinoma patients after radiofrequency ablation treatment. Proteomics. 2005;5:4287–95.
Honda M, Kaneko S, Kawai H, Shirota Y, Kobayashi K. Differential gene expression between chronic hepatitis B and C hepatic lesion. Gastroenterology. 2001;120:955–66.
Kim W, Oe Lim S, Kim JS, Ryu YH, Byeon JY, Kim HJ, et al. Comparison of proteome between hepatitis B virus- and hepatitis C virus-associated hepatocellular carcinoma. Clin Cancer Res. 2003;9:5493–500.
Koike K. Steatosis, liver injury, and hepatocarcinogenesis in hepatitis C viral infection. J Gastroenterol. 2009;44:82–8.
Lok AS, Lai CL. Alpha-fetoprotein monitoring in Chinese patients with chronic hepatitis B virus infection: role in the early detection of hepatocellular carcinoma. Hepatology. 1989;9:110–5.
Markiewski MM, Mastellos D, Tudoran R, DeAngelis RA, Strey CW, Franchini S, et al. C3a and C3b activation products of the third component of complement (C3) are critical for normal liver recovery after toxic injury. J Immunol. 2004;173:747–54.
Oka H, Kurioka N, Kim K, Kanno T, Kuroki T, Mizoguchi Y, et al. Prospective study of early detection of hepatocellular carcinoma in patients with cirrhosis. Hepatology. 1990;12:680–7.
Tanaka N, Horiuchi A, Yamaura T, Komatsu M, Tanaka E, Kiyosawa K. Efficacy and safety of 6-month iron reduction therapy in patients with hepatitis C virus-related cirrhosis: a pilot study. J Gastroenterol. 2007;42:49–55.
Tsamandas AC, Antonacopoulou A, Kalogeropoulou C, Tsota I, Zabakis P, Giannopoulou E, et al. Oval cell proliferation in cirrhosis in rats. An experimental study. Hepatol Res. 2007;37:755–64.
Acknowledgments
We thank Mr. Hiroyuki Nakao for assistance with the statistical analyses. We also thank Ms. Yuko Morinaga for her technical assistance. This work was supported in part by a grant-in-aid from the Collaboration of Regional Entities for the Advancement of Technological Excellence (CREATE) from the Japan Science and Technology Agency, a grant (no. CA87982) from the United States National Institutes of Health, and a grant-in-aid (Research on Hepatitis and BSE) from the Ministry of Health, Labour and Welfare of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kanmura, S., Uto, H., Sato, Y. et al. The complement component C3a fragment is a potential biomarker for hepatitis C virus-related hepatocellular carcinoma. J Gastroenterol 45, 459–467 (2010). https://doi.org/10.1007/s00535-009-0160-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-009-0160-5