Abstract
Background
Uncontrolled studies show fatigue, anorexia, depression, and mortality are associated with low testosterone in men with cancer. Testosterone replacement improves quality of life and diminishes fatigue in patients with non-cancer conditions. The primary objective was to evaluate the effect of testosterone replacement on fatigue in hypogonadal males with advanced cancer, by the Functional Assessment of Chronic Illness Therapy-Fatigue subscale (FACIT-Fatigue) at day 29.
Methods
This is a randomized, double-blinded placebo-controlled trial. Outpatients with advanced cancer, bioavailable testosterone (BT) <70 ng/dL and fatigue score >3/10 on the Edmonton Symptom Assessment Scale were eligible. Intra-muscular testosterone or sesame seed oil placebo was administered every 14 days to achieve BT levels 70–270 ng/dL.
Results
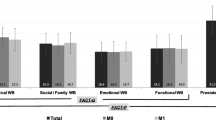
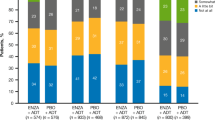
Sixteen placebo and 13 testosterone-treated subjects were evaluable. No statistically significant difference was found for FACIT-fatigue scores between arms (−2 ± 12 for placebo, 4 ± 8 for testosterone, p = 0.11). Sexual Desire Inventory score (p = 0.054) and performance status (p = 0.02) improved in the testosterone group. Fatigue subscale scores were significantly better (p = 0.03) in those treated with testosterone by day 72.
Conclusions
Four weeks of intramuscular testosterone replacement in hypogonadal male patients with advanced cancer did not significantly improve quality of life. Larger studies of longer duration are warranted.

Similar content being viewed by others
References
Strasser F, Palmer JL, Schover LR et al (2006) The impact of hypogonadism and autonomic dysfunction on fatigue, emotional function, and sexual desire in male patients with advanced cancer: a pilot study. Cancer 107:2949–2957
Burney BO, Hayes TG, Smiechowska J et al (2012) Low testosterone levels and increased inflammatory markers in patients with cancer and relationship with cachexia. J Clin Endocrinol Metab 97:700–709
Chlebowski RT, Heber D (1982) Hypogonadism in male patients with metastatic cancer prior to chemotherapy. Cancer Res 42:2495–2498
Del Fabbro E, Hui D, Nooruddin ZI et al (2010) Associations among hypogonadism, C-reactive protein, symptom burden, and survival in male cancer patients with cachexia: a preliminary report. J Pain Symptom Manag 39:1016–1024
Rabkin JG, Wagner GJ, Rabkin R (2000) A double-blind, placebo-controlled trial of testosterone therapy for HIV-positive men with hypogonadal symptoms. Arch Gen Psychiatry 57:141–147
Patrick DL, Ferketich SL, Frame PS et al (2004) National Institutes of Health State-of-the-Science Conference Statement: Symptom management in cancer: pain, depression, and fatigue, July 15–17, 2002. National Institutes of Health State-of-the-Science Panel. J Natl Cancer Inst Monogr 32:9–16
Bhasin S, Cunningham GR, Hayes FJ et al (2006) Testosterone therapy in adult men with androgen deficiency syndromes: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 91:1995–2010
Garcia JM, Li H, Mann D, Epner D et al (2006) Hypogonadism in male patients with cancer. Cancer 106:2583–2591
Rajagopal A, Vassilopoulou-Sellin R, Palmer JL, Kaur G, Bruera E (2004) Symptomatic hypogonadism in male survivors of cancer with chronic exposure to opioids. Cancer 100:851–858
Gerl A, Muhlbayer D, Hansmann G, Mraz W, Hiddemann W (2001) The impact of chemotherapy on Leydig cell function in long term survivors of germ cell tumors. Cancer 91:1297–1303
Dev R, Del Fabbro E, Bruera E (2007) Association between megestrol acetate treatment and symptomatic adrenal insufficiency with hypogonadism in male patients with cancer. Cancer 110:1173–1177
Malkin CJ, Pugh PJ, Morris PD, Kerry KE, Jones RD, Jones TH, Channer KS (2004) Testosterone replacement in hypogonadal men with angina improves ischaemic threshold and quality of life. Heart 90:871–878
Chiang HS, Cho SL, Lin YC, Hwang TI (2009) Testosterone gel monotherapy improves sexual function of hypogonadal men mainly through restoring erection: evaluation by IIEF score. Urology 73:762–766
Cherrier MM, Asthana S, Plymate S, Baker L, Matsumoto AM, Peskind E, Raskind MA, Brodkin K, Bremner W, Petrova A, LaTendresse S, Craft S (2001) Testosterone supplementation improves spatial and verbal memory in healthy older men. Neurology 57:80–88
Bhasin S, Storer TW, Javanbakht M, Berman N, Yarasheski KE, Phillips J, Dike M, Sinha-Hikim I, Shen R, Hays RD, Beall G (2000) Testosterone replacement and resistance exercise in HIV-infected men with weight loss and low testosterone levels. JAMA 283:763–770
Morley JE, Charlton E, Patrick P, Kaiser FE, Cadeau P, McCready D, Perry HM (2000) Validation of a screening questionnaire for androgen deficiency in aging males. Metabolism 49:1239–1242
Bruera E, Kuehn N, Miller MJ et al (1991) The Edmonton Symptom Assessment System (ESAS). J Palliat Care 7:6–9
Chang VT, Hwang SS, Feuerman M (2000) Validation of the Edmonton Symptom Assessment Scale. Cancer 88:2164–2171
Cella D, Tulsky DS, Gray G et al (1993) The functional assessment of cancer therapy scale: development and validation of the general measure. J Clin Oncol 11:570–579
Ribaudo JM, Cella D, Hahn EA, Lloyd SR, Tchekmedyian NS et al (2000) Re-validation and shortening of the Functional Assessment of Anorexia/Cachexia Therapy (FAACT) questionnaire. Qual Life Res 9:1137–1146
Johnston M, Pollard B, Hennessey P (2000) Construct validation of the hospital anxiety and depression scale with clinical populations. J Psychosomatic Res 48:579–584
Spector IP, Carey MP, Steinberg L (1996) The Sexual Desire Inventory: development, factor structure, and evidence of reliability. J Sex Marital Ther 22:175–190
Barry MJ, Fowler FJ Jr, O'Leary MP, Bruskewitz RC et al (1992) for The Measurement Committee of the American Urological Association, The American Urological Association symptom index for benign prostatic hyperplasia. J Urol 148:1549–1557
American Thoracic Society (2002) ATS Statement: Guidelines for the Six-Minute Walk Test. Am J Respir Crit Care Med 166:111–117
Sousa N, Sampaio J (2005) Effects of progressive strength training on the performance of the Functional Reach Test and the Timed Get-Up-and-Go Test. Am J Hum Biol 17:746–751
Wheeler MJ (1995) The determination of bioavailable testosterone. Ann Clin Biochem 32:345–357
Oken MM, Creech RH, Tormey DC et al (1982) Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J ClinOncol 5:649–655
Lai JS, Beaumont JL, Ogale S, Brunetta P, Cella D (2011) Validation of the functional assessment of chronic illness therapy-fatigue scale in patients with moderately to severely active systemic lupus erythematosus, participating in a clinical trial. J Rheumatol 38:672–679
Cella D, Hahn EA, Dineen K (2002) Meaningful change in cancerspecific quality of life scores: differences between improvement and worsening. Qual Life Res 11:207–221
Yost KJ, Sorensen MV, Hahn EA, Glendenning GA, Gnanasakthy A, Cella D (2005) Using multiple anchor- and distribution-based estimates to evaluate clinically meaningful change on the Functional Assessment of Cancer Therapy-Biologic Response Modifiers (FACT-BRM) instrument. Value Health 8:117–127
Cella D, Eton DT, Lai JS et al (2002) Combining anchor and distribution-based methods to derive minimal clinically important differences on the Functional Assessment ofCancer Therapy (FACT) Anemia and Fatigue scales. J PainSymptom Manage 24:547–561
Lindau ST, Gavrilova N (2010) Sex, health, and years of sexually active life gained due to good health: evidence from two US population based cross sectional surveys of ageing. BMJ 9:340–c810
Garcia JM, Li H, Mann D, Epner D, Hayes TG, Marcelli M, Cunningham GR (2006) Hypogonadism in male patients with cancer. Cancer 106:2583–2591
Shafqat A, Einhorn LH, Hanna N, Sledge GW, Hanna A, Juliar BE, Monahan P, Bhatia S (2005) Screening studies for fatigue and laboratory correlates in cancer patients undergoing treatment. Ann Oncol 16:1545–1550
Coviello AD, Kaplan B, Lakshman KM, Chen T, Singh AB, Bhasin S (2008) Effects of graded doses of testosterone on erythropoiesis in healthy young and older men. J Clin Endocrinol Metab 93:914–919
Munch TN, Zhang T, Willey J, Palmer JL, Bruera E (2005) The association between anemia and fatigue in patients with advanced cancer receiving palliative care. J Palliat Med 8:1144–1149
Minton O, Strasser F, Radbruch L, Stone P (2012) Identification of factors associated with fatigue in advanced cancer: a subset analysis of the European palliative care research collaborative computerized symptom assessment data set. J Pain Symptom Manage 43(2):226–235
Mercadante S, Ferrera P, Villari P, David F, Giarratano A, Riina S (2009) Effects of red blood cell transfusion on anemia-related symptoms in patients with cancer. J Palliat Med 12:60–63
Trutschnigg B, Kilgour RD, Reinglas J, Rosenthall L, Hornby L, Morais JA, Vigano A (2008) Precision and reliability of strength (Jamar vs. Biodex handgrip) and body composition (dual-energy X-ray absorptiometry vs. bioimpedance analysis) measurements in advanced cancer patients. Appl Physiol Nutr Metab 33:1232–1239
MacDonald AJ, Greig CA, Baracos V (2011) The advantages and limitations of cross-sectional body composition analysis. Curr Opin Support Palliat Care 5:342–349
Eggertson L (2011) Brouhaha erupts over testosterone-testing advertising campaign. CMAJ 183:1161–1162
Marks LS, Mazer NA, Mostaghel E et al (2006) Effect of testosterone replacement therapy on prostate tissue in men with late-onset hypogonadism: a randomized controlled trial. JAMA 296:2351–2361
Rhoden EL, Morgentaler A (2003) Testosterone replacement therapy in hypogonadal men at high risk for prostate cancer: results of 1 year of treatment in men with prostatic intraepithelial neoplasia. J Urol 170:2348–2351
Acknowledgments
Funding
This work was supported by a grant from the American Cancer Society# PEP-08-299-01-PC1 to E. Del Fabbro.
E. Bruera is supported in part by a National Institutes of Health grant numbers RO1NR010162-01A1, RO1CA1222292.01, and RO1CA124481-01.
J. Garcia is supported in part by the Dept of Veterans Affairs MERIT grants (I01-BX000507 and I01 CX000174) and the NIA (T32AG000183 and AG040583).
D. Hui is supported in part by the Clinician Investigator Program, Royal College of Physicians and Surgeons of Canada.
Disclosure
The authors have declared no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
E. Del Fabbro and J.M Garcia contributed equally to this work.
Rights and permissions
About this article
Cite this article
Del Fabbro, E., Garcia, J.M., Dev, R. et al. Testosterone replacement for fatigue in hypogonadal ambulatory males with advanced cancer: a preliminary double-blind placebo-controlled trial. Support Care Cancer 21, 2599–2607 (2013). https://doi.org/10.1007/s00520-013-1832-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-013-1832-5