Abstract
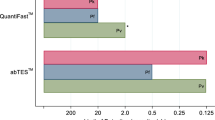
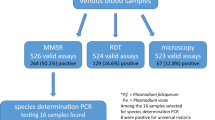
Microscopy and rapid diagnostic tests (RDTs) are the techniques commonly used for malaria diagnosis but they are usually insensitive at very low levels of parasitemia. Nested PCR is commonly used as a reference technique in the diagnosis of malaria due to its high sensitivity and specificity. However, it is a cumbersome assay only available in reference centers. We evaluated a new nested PCR-based assay, BIOMALAR kit (Biotools B&M Labs, Madrid, Spain) which employs ready-to-use gelled reagents and allows the identification of the main four species of Plasmodium. Blood samples were obtained from patients with clinical suspicion of malaria. A total of 94 subjects were studied. Fifty-two (55.3 %) of them were malaria-infected subjects corresponding to 48 cases of Plasmodium falciparum, 1 Plasmodium malariae, 2 Plasmodium vivax, and 1 Plasmodium ovale. The performance of the BIOMALAR test was compared with microscopy, rapid diagnostic test (RDT) (BinaxNOW® Malaria) and real-time quantitative PCR (qPCR). The BIOMALAR test showed a sensitivity of 98.1 % (95 % confidence interval [CI], 89.7–100), superior to microscopy (82.7 % [95 % CI, 69.7–91.8]) and RDT (94.2 % [95 % CI, 84.1–98.8]) and similar to qPCR (100 % [95 % CI, 93.2–100]). In terms of specificity, the BIOMALAR assay showed the same value as microscopy and qPCR (100 % [95 % CI, 93.2–100]). Nine subjects were submicroscopic carriers of malaria. The BIOMALAR test identified almost all of them (8/9) in comparison with RDT (6/9) and microscopy (0/9). In conclusion, the BIOMALAR is a PCR-based assay easy to use with an excellent performance and especially useful for diagnosis submicroscopic malaria.
Similar content being viewed by others
References
Alam MS, Mohon AN, Mustafa S, Khan WA, Islam N, Karim MJ, Khanum H, Sullivan DJ Jr, Haque R (2011) Real-time PCR assay and rapid diagnostic tests for the diagnosis of clinically suspected malaria patients in Bangladesh. Malar J 10:175
Banoo S, Bell D, Bossuyt P, Herring A, Mabey D, Poole F, Smith PG, Sriram N, Wongsrichanalai C, Linke R, O'Brien R, Perkins M, Cunningham J, Matsoso P, Nathanson CM, Olliaro P, Peeling RW, Ramsay A, TDR Diagnostics Evaluation Expert Panel (2010) Evaluation of diagnostic tests for infectious diseases: general principles. Nat Rev Microbiol 8(Suppl 12):17–29
Berry A, Benoit-Vical F, Fabre R, Cassaing S, Magnaval JF (2008) PCR-based methods to the diagnosis of imported malaria. Parasite 15(3):484–488
Cheesbrough M (1987) Medical laboratory manual for tropical countries, vol I, 2nd edn. University Press, Cambridge
Cotter C, Sturrock HJ, Hsiang MS, Liu J, Phillips AA, Hwang J, Gueye CS, Fullman N, Gosling RD, Feachem RG (2013) The changing epidemiology of malaria elimination: new strategies for new challenges. Lancet 382(9895):900–911
Golassa L, Enweji N, Erko B, Aseffa A, Swedberg G (2013) Detection of a substantial number of sub-microscopic Plasmodium falciparum infections by polymerase chain reaction: a potential threat to malaria control and diagnosis in Ethiopia. Malar J 12:352
Laouenan F, Monsalve LG, Goiriena A, Agirregabiria M, Ruano-Lopez JM (2012) A self-contained diagnostic platform for DNA concentration, elution, and qPCR inside a LabCard with stored reagents. Procedia Eng 47:1484–1490
Mohammed AH, Salih MM, Elhassan EM, Mohmmed AA, Elzaki SE, El-Sayed BB, Adam I (2013) Submicroscopic Plasmodium falciparum malaria and low birth weight in an area of unstable malaria transmission in Central Sudan. Malar J 12:172
Moody A (2002) Rapid diagnostic tests for malaria parasites. Clin Microbiol Rev 15(1):66–78
Nilles EJ, Arguin PM (2012) Imported malaria: an update. Am J Emerg Med 30(6):972–980
Okell LC, Ghani AC, Lyons E, Drakeley CJ (2009) Submicroscopic infection in Plasmodium falciparum-endemic populations: a systematic review and meta-analysis. J Infect Dis 200(10):1509–1517
Okell LC, Bousema T, Griffin JT, Ouédraogo AL, Ghani AC, Drakeley CJ (2012) Factors determining the occurrence of submicroscopic malaria infections and their relevance for control. Nat Commun 3:1237
Oliveira DA, Shi YP, Oloo AJ, Boriga DA, Nahlen BL, Hawley WA, Holloway BP, Lal AA (1996) Field evaluation of a polymerase chain reaction-based nonisotopic liquid hybridization assay for malaria diagnosis. J Infect Dis 173(5):1284–1287
Padley D, Moody AH, Chiodini PL, Saldanha J (2003) Use of a rapid, single-round, multiplex PCR to detect malarial parasites and identify the species present. Ann Trop Med Parasitol 97(2):131–137
Ramírez-Olivencia G, Rubio JM, Rivas P, Subirats M, Herrero MD, Lago M, Puente S (2012) Imported submicroscopic malaria in Madrid. Malar J 11:324
Rogers WO (2007) Plasmodium and Babesia. In: Murray PR, Baron EJ, Jorgensen JH, Landry ML, Pfaller MA (eds) Manual of clinical microbiology, vol 2, 9th edn. ASM Press, Washington DC, pp 2040–2056
Roper C, Elhassan IM, Hviid L, Giha H, Richardson W, Babiker H, Satti GM, Theander TG, Arnot DE (1996) Detection of very low level Plasmodium falciparum infections using the nested polymerase chain reaction and a reassessment of the epidemiology of unstable malaria in Sudan. Am J Trop Med Hyg 54(4):325–331
Rougemont M, Van Saanen M, Sahli R, Hinrikson HP, Bille J, Jaton K (2004) Detection of four Plasmodium species in blood from humans by 18S rRNA gene subunit-based and species-specific real-time PCR assays. J Clin Microbiol 42(12):5636–5643
Shokoples SE, Ndao M, Kowalewska-Grochowska K, Yanow SK (2009) Multiplexed real-time PCR assay for discrimination of Plasmodium species with improved sensitivity for mixed infections. J Clin Microbiol 47(4):975–980
Snounou G, Viriyakosol S, Jarra W, Thaithong S, Brown KN (1993) Identification of the four human malaria parasite species in field samples by the polymerase chain reaction and detection of a high prevalence of mixed infections. Mol Biochem Parasitol 58(2):283–292
Sun Y, Høgberg J, Christine T, Florian L, Monsalve LG, Rodriguez S, Cao C, Wolff A, Ruano-Lopez JM, Bang DD (2013) Pre-storage of gelified reagents in a lab-on-a-foil system for rapid nucleic acid analysis. Lab Chip 13(8):1509–1514
WHO (2013) World malaria report 2013. World Health Organization, Geneve, Switzerland
Wilson ML (2012) Malaria rapid diagnostic tests. Clin Infect Dis 54(11):1637–1641
Wongsrichanalai C, Barcus MJ, Muth S, Sutamihardja A, Wernsdorfer WH (2007) A review of malaria diagnostic tools: microscopy and rapid diagnostic test (RDT). Am J Trop Med Hyg 77(Suppl 6):119–127
Acknowledgments
We are indebted to the staff of the Tropical Medicine Department at Hospital Carlos III for their help with the collection of data.
Conflict of interest
All authors have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Iglesias, N., Subirats, M., Trevisi, P. et al. Performance of a new gelled nested PCR test for the diagnosis of imported malaria: comparison with microscopy, rapid diagnostic test, and real-time PCR. Parasitol Res 113, 2587–2591 (2014). https://doi.org/10.1007/s00436-014-3911-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-014-3911-z