Abstract
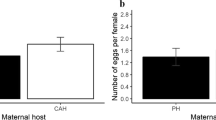
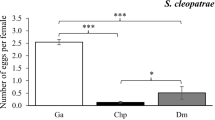
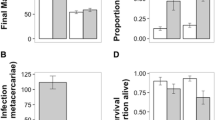
Host condition can influence both the nutritive resources available to parasites and the strength of host defences. Since these factors are likely to be correlated, it is unclear whether parasites would be more successful on hosts in good, intermediate or poor conditions. For more complex parasites, like fleas, where larvae depend on adults to extract and make available some essential host resources, host condition can act at two levels. First, it can affect the investment of females into eggs, and second, it can influence offspring growth. In a two-step experiment, we first let female hen fleas Ceratophyllus gallinae feed on nestlings of reduced, control or enlarged great tit Parus major broods and secondly used the blood from these nestlings as a food source for flea larvae reared in the laboratory. We then assessed the effect of brood size manipulation on reproductive investment and survival of female fleas, and on survival, developmental time, mass and size of pre-imago larvae and adults of the first generation. Although host condition, measured as body mass controlled for body size, was significantly influenced by brood size manipulation, it did not affect the female fleas' reproductive investment and survival. Larvae fed with blood from nestlings of reduced broods lived longer, however, than larvae fed on blood from enlarged or control broods. Additionally, F1 adults grew shorter tibiae when their mother had fed on hosts of reduced broods. The finding that brood size manipulation influenced parasite reproduction suggests that it affected nutritive resources and/or host defence, but the precise mechanism or balance between the two requires further investigation.

Similar content being viewed by others
References
Berthouly A, Cassier A, Richner H (2008) Carotenoid-induced maternal effects interact with ectoparasite burden and brood size to shape the trade-off between growth and immunity in nestling great tits. Funct Ecol 22(5):854–863. doi:10.1111/j.1365-2435.2008.01439.x
Bize P, Jeanneret C, Klopfenstein A, Roulin A (2008) What makes a host profitable? Parasites balance host nutritive resources against immunity. Am Nat 171(1):107–118. doi:10.1086/523943
Brinkhof MWG, Heeb P, Kolliker M, Richner H (1999) Immunocompetence of nestling great tits in relation to rearing environment and parentage. Proc R Soc Lond B Biol Sci 266(1435):2315–2322
Christe P, Møller AP, de Lope F (1998) Immunocompetence and nestling survival in the house martin: the tasty chick hypothesis. Oikos 83(1):175–179
Christe P, Giorgi MS, Vogel P, Arlettaz R (2003) Differential species-specific ectoparasitic mite intensities in two intimately coexisting sibling bat species: resource-mediated host attractiveness or parasite specialization? J Anim Ecol 72(5):866–872
Coll M, Guershon M (2002) Omnivory in terrestrial arthropods: mixing plant and prey diets. Annu Rev Entomol 47:267–297
Dryden MW, Smith V (1994) Cat flea (Siphonaptera, Pulicidae) cocoon formation and development of naked flea pupae. J Med Entomol 31(2):272–277
Du Feu CR (1987) Some observations on fleas emerging from tit nestboxes. Ringing Migr 8:123–128
Griffiths R, Double MC, Orr K, Dawson RJG (1998) A DNA test to sex most birds. Mol Ecol 7(8):1071–1075
Hinkle NC, Koehler PG, Kern WH, Patterson RS (1991) Hematophagous strategies of the cat flea (Siphonaptera: Pulicidae). Fla Entomol 74(3):377–385
Hsu MH, Hsu YC, Wu WJ (2002) Consumption of flea faeces and eggs by larvae of the cat flea, Ctenocephalides felis. Med Vet Entomol 16(4):445–447
Humphries DA (1968) The host-finding behaviour of the hen flea Ceratophyllus gallinae (Schrank) (Siphonaptera). Parasitology 58:403–414
Krasnov BR (2008) Functional and evolutionary ecology of fleas: a model for ecological parasitology. Cambridge University Press, New York, pp 206–216
Krasnov BR, Khokhlova IS, Fielden LJ, Burdelova NV (2001) Development rates of two Xenopsylla flea species in relation to air temperature and humidity. Med Vet Entomol 15(3):249–258
Krasnov BR, Sarfati M, Arakelyan MS, Khokhlova IS, Burdelova NI, Degen AA (2003) Host specificity and foraging efficiency in blood-sucking parasite: feeding patterns of the flea Parapulex chephrenis on two species of desert rodents. Parasitol Res 90(5):393–399. doi:10.1007/s00436-003-0873-y
Krasnov BR, Khokhlova IS, Arakelyan MS, Degen AA (2005) Is a starving host tastier? Reproduction in fleas parasitizing food-limited rodents. Funct Ecol 19(4):625–631. doi:10.1111/j.1365-2435.2005.01015.x
Krist AC, Jokela J, Wiehn J, Lively CM (2004) Effects of host condition on susceptibility to infection, parasite developmental rate, and parasite transmission in a snail-trematode interaction. J Evol Biol 17(1):33–40. doi:10.1046/j.1420-9101.2003.00661.x
Lawrence W, Foil LD (2002) The effects of diet upon pupal development and cocoon formation by the cat flea (Siphonaptera: Pulicidae). J Vector Ecol 27(1):39–43
Le Lagadec MD, Chown SL, Scholtz CH (1998) Desiccation resistance and water balance in southern African keratin beetles (Coleoptera, Trogidae): the influence of body size and habitat. J Comp Physiol B Biochem Syst Environ Physiol 168(2):112–122
Lehane MJ (1991) Biology of blood-sucking insects. HarperCollins Academic, London, pp 87–96; pp 98–102
Losdat S, Helfenstein F, Gaude B, Richner H (2010) Effects of sibling competition and male carotenoid supply on offspring condition and oxidative stress. Behav Ecol 21(6):1271–1277
Lourenço S, Palmeirim JM (2008) Which factors regulate the reproduction of ectoparasites of temperate-zone cave-dwelling bats? Parasitol Res 104(1):127–134. doi:10.1007/s00436-008-1170-6
Marshall AG (1981) The ecology of ectoparasitic insects. Academic Press, London, pp 132–133
Mirth CK, Riddiford LM (2007) Size assessment and growth control: how adult size is determined in insects. Bioessays 29(4):344–355. doi:10.1002/bies.20552
Møller AP (1997) Parasitism and the evolution of host life history. In: Clayton DH, Moore J (eds) Host-parasite evolution: general principles and avian models. Oxford University Press, Oxford, pp 105–127
Mousseau TA, Fox CW (1998) The adaptive significance of maternal effects. Trends Ecol Evol 13(10):403–407
Nelson WA (1984) Effects of nutrition of animals on their ectoparasites. J Med Entomol 21(6):621–635
Neuenschwander S, Brinkhof MWG, Kölliker M, Richner H (2003) Brood size, sibling competition, and the cost of begging in great tits (Parus major). Behav Ecol 14(4):457–462
Nijdam E, Delezie E, Lambooij E, Nabuurs MJA, Decuypere E, Stegeman JA (2005) Feed withdrawal of broilers before transport changes plasma hormone and metabolite concentrations. Poult Sci 84(7):1146–1152
Nijhout HF (1975) Threshold size for metamorphosis in the tobacco hornworm, Manduca sexta (L.). Biol Bull 149(1):214–225
Nijhout HF, Williams CM (1974) Control of molting and metamorphosis in tobacco hornworm, Manduca sexta (L.)—Growth of last-instar larva and decision to pupate. J Exp Biol 61(2):481–491
Parmentier HK, Reilingh GD, Nieuwland MGB (1998) Kinetic and immunohistochemical characteristics of mitogen-induced cutaneous hypersensitivity in chickens selected for antibody responsiveness. Vet Immunol Immunopathol 66(3–4):367–376
Pinheiro J, Bates D, DebRoy S, Deepayan S, R Development Core Team (2011) nlme: linear and nonlinear mixed effects models. R package version 3.1-100. http://cran.r-project.org/web/packages/nlme/index.html
Poulin R (1998) Evolutionary ecology of parasites. Chapman and Hall, London, pp 90–110
R Development Core Team (2011) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.R-project.org
Reimchen TE, Nosil P (2001) Ecological causes of sex-biased parasitism in three spine stickleback. Biol J Linn Soc 73(1):51–63. doi:10.1006/bijl.2001.0523
Richner H, Oppliger A, Christe P (1993) Effect of an ectoparasite on reproduction in great tits. J Anim Ecol 62(4):703–710
Rossin MA, Poulin R, Timi JT, Malizia AI (2005) Causes of inter-individual variation in reproductive strategies of the parasitic nematode Graphidioides subterraneus. Parasitol Res 96(5):335–339. doi:10.1007/s00436-005-1400-0
Rossiter MC (1991) Environmentally-based maternal effects—a hidden force in insect population-dynamics. Oecologia 87(2):288–294
Rothschild ML, Clay T (1952) Fleas, flukes and cuckoos; a study of bird parasites. Collins, London, pp 71–72; pp 111–113
Roulin A, Ducrest A-L, Dijkstra C (1999) Effect of brood size manipulations on parents and offspring in the barn owl Tyto alba. Ardea 87:91–100
Roulin A et al (2003) Which chick is tasty to parasites? The importance of host immunology vs. parasite life history. J Anim Ecol 72(1):75–81
Sarfati M, Krasnov BR, Ghazaryan L, Khokhlova IS, Fielden LJ, Degen AA (2005) Energy costs of blood digestion in a host-specific haematophagous parasite. J Exp Biol 208(13):2489–2496. doi:10.1242/jeb.01676
Shaw DJ, Dobson AP (1995) Patterns of macroparasite abundance and aggregation in wildlife populations: a quantitative review. Parasitology 111:S111–S133
Sheldon BC, Verhulst S (1996) Ecological immunology: costly parasite defences and trade-offs in evolutionary ecology. Trends Ecol Evol 11(8):317–321
Silverman J, Appel AG (1994) Adult cat flea (Siphonaptera, Pulicidae) excretion of host blood proteins in relation to larval nutrition. J Med Entomol 31(2):265–271
Strenger A (1973) Zur Ernährungsbiologie der Larve von Ctenocephalides felis felis. Zool Jahrb Syst 100:64–80
Tripet F, Richner H (1997) Host responses to ectoparasites: food compensation by parent blue tits. Oikos 78(3):557–561
Tripet F, Richner H (1999) Dynamics of hen flea Ceratophyllus gallinae subpopulations in blue tit nests. J Insect Behav 12(2):159–174
Tripet F, Jacot A, Richner H (2002) Larval competition affects the life histories and dispersal behavior of an avian ectoparasite. Ecology 83(4):935–945
Tschirren B, Bischoff LL, Saladin V, Richner H (2007) Host condition and host immunity affect parasite fitness in a bird-ectoparasite system. Funct Ecol 21(2):372–378. doi:10.1111/j.1365-2435.2007.01235.x
Tseng M (2006) Interactions between the parasite's previous and current environment mediate the outcome of parasite infection. Am Nat 168(4):565–571
Verhulst S, Nilsson JA (2008) The timing of birds' breeding seasons: a review of experiments that manipulated timing of breeding. Philos Trans R Soc B-Biol Sci 363(1490):399–410. doi:10.1098/rstb.2007.2146
Acknowledgements
We are grateful to V. Saladin for molecular sexing of nestlings and to M. Coslovsky and M. Rueesch for helpful comments on the manuscript. We also wish to thank M. Coslovsky for kindly allowing us to use his nest boxes. The work was approved by the veterinary office of the Canton Berne, Switzerland (permit number 22/10) and financially supported by the Swiss National Science Foundation (grant number 3100A0-122566/1).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rueesch, S., Lemoine, M. & Richner, H. Ectoparasite reproductive performance when host condition varies. Parasitol Res 111, 1193–1203 (2012). https://doi.org/10.1007/s00436-012-2953-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-012-2953-3