Abstract
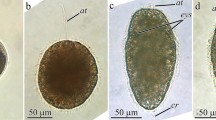
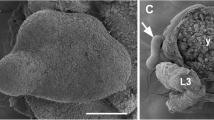
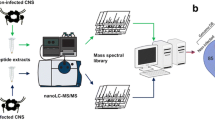
The widespread reports of malformed frogs have sparked interest worldwide to try and determine the causes of such malformations. Ribeiroia ondatrae is a digenetic trematode, which has been implicated as one such cause, as this parasite encysts within the developing tadpole hind limb bud and inguinal region causing dramatic limb malformations. Currently, the mechanisms involved in parasite-induced limb deformities remain unclear. We sought to investigate whether the level of retinoic acid (RA), a morphogenetic factor known to play a critical role in limb bud formation, is altered by the presence of R. ondatrae within the infected tadpole. Alteration of RA levels within the limb bud caused by the presence of the parasite may be achieved in three ways. First, metacercariae are actively secreting RA; second, cercariae, upon entering the limb/inguinal region, may release a large amount of RA; finally, the metacercariae may induce either an increase in the synthesis or a decrease in the degradation of the host’s endogenous retinoic acid levels. Here, we show through high performance liquid chromatography and mass spectrometry that limb bud tissue of Lithobates sylvaticus, which has been parasitised, contains 70% more RA compared to the unparasitised control. Furthermore, parasites that have encysted within the limb buds appear to contain substantially less RA (56%) than the free swimming cercariae (defined as the infectious stage of the parasite). Taken together, these data illustrate for the first time that encystment of R. ondatrae leads to an increase in RA levels in the tadpole limb bud and may offer insight into the mechanisms involved in parasite-induced limb deformities.





Similar content being viewed by others
References
Biron DG, Marche L, Ponton F, Loxdale HD, Galeotti N, Renault L, Joly C, Thomas F (2005) Behavioural manipulation in a grasshopper harbouring hairworm: a proteomics approach. P Roy Soc B Biol Sci 272:2117–2126
Blaustein AR, Johnson PT (2003) The complexity of deformed amphibians. Front Ecol Environ 1:87–94
Bryant SV, Gardiner DM (1992) Retinoic acid, local cell–cell interactions, and pattern-formation in vertebrate limbs. Dev Biol 152:1–25
Collins JP, Storfer A (2003) Global amphibian declines: sorting the hypotheses. Divers Distrib 9:89–98
Dalzell BJ, Sutherland DR (1998) Possibilities of Manodistomum syntomentera as a cause of deformities in Rana pipiens. Univ Wis Crosse J Undergrad Res 1:28–32
Degitz SJ, Kosian PA, Makynen EA, Jensen KM, Ankley GT (2000) Stage- and species-specific developmental toxicity of all-trans retinoic acid in four native North American ranids and Xenopus laevis. Toxicol Sci 57:264–274
Dmetrichuk JM, Carlone RL, Jones TRB, Vesprini ND, Spencer GE (2008) Detection of endogenous retinoids in the molluscan CNS and characterization of the trophic and tropic actions of 9-cis retinoic acid on isolated neurons. J Neurosci 28:13014–13024
Elinson RP, Walton Z, Lee SY, Nath K (2008) Conservation in a frog of the retinoic acid requirement for forelimb initiation. Dev Biol 319:498
Fried B (1997) An overview of the biology of trematodes. In: Fried B, Graczyk TK (eds) Advances in trematode biology. CRC, Boca Raton, pp 12–13
Gardiner D, Ndayibagira A, Grun F, Blumberg B (2003) Deformed frogs and environmental retinoids. Pure Appl Chem 75:2263–2273
Gosner KL (1960) A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16:183–190
Huffman JE, Fried B (1990) Echinostoma and echinostomiasis. Adv Parasitol 29:215–269
Johnson PTJ, Sutherland DR (2003) Amphibian deformities and Ribeiroia infection: an emerging helminthiasis. Trends Parasitol 19:332–335
Johnson PTJ, Lunde KB, Ritchie EG, Launer AE (1999) The effect of trematode infection on amphibian limb development and survivorship. Science 284:802–804
Johnson PTJ, Lunde KB, Thurman EM, Ritchie EG, Wray SN, Sutherland DR, Kapfer JM, Frest TJ, Bowerman J, Blaustein AR (2002) Parasite (Ribeiroia ondatrae) infection linked to amphibian malformations in the western United States. Ecol Monogr 72:151–168
Johnson PTJ, Sutherland DR, Kinsella JM, Lunde KB (2004) Review of the trematode genus Ribeiroia (Psilostomidae): Ecology, life history and pathogenesis with special emphasis on the amphibian malformation problem. Adv Parasitol 57:191–253
Lee GS, Kochhar DM, Collins MD (2004) Retinoid-induced limb malformations. Curr Pharm Des 10:2657–2699
Lewandoskia M, Mackem S (2009) Limb development: the rise and fall of retinoic acid. Curr Biol 19:R558–R561
Maden M (1983) The effect of vitamin-a on limb regeneration in Rana temporaria. Dev Biol 98:409–416
Maden M (1996) Retinoic acid in development and regeneration. J Biosci 21:299–312
Maden M (1999) Heads or tails? Retinoic acid will decide. Bioessays 21:809–812
Maden M (2007) Retinoic acid in the development, regeneration and maintenance of the nervous system. Nat Rev Neurosci 8:755–765
Maden M, Hind M (2003) Retinoic acid, a regeneration-inducing molecule. Dev Dyn 226:237–244
Maden M, Sonneveld E, van der Saag PT, Gale E (1998) The distribution of endogenous retinoic acid in the chick embryo: implications for developmental mechanisms. Development 125:4133–4144
Mark M, Ghyselinck NB, Chambon P (2009) Function of retinoic acid receptors during embryonic development. Nucl Recept Signal 7:e002
Meteyer CU, Loeffler IK, Fallon JF, Converse KA, Green E, Helgen JC, Kersten S, Levey R, Eaton-Poole L, Burkhart JG (2000) Hind limb malformations in free-living northern leopard frogs (Rana pipiens) from Maine, Minnesota, and Vermont suggest multiple etiologies. Teratology 62:151–171
Moore J (2002) Behavioral alterations and parasite transmission. In: May RM, Harvey PH (eds) Parasites and the behavior of animals. Oxford University Press, Oxford, pp 35–89
Olsen OW (1974) Animal parasites: their life cycles and ecology, 3rd edn. University Park Press, Baltimore
Rajakaruna RS, Piyatissa PMJR, Jayawardena UA, Navaratne AN, Amerasinghe PH (2008) Trematode infection induced malformations in the common hourglass treefrogs. J Zool 275:89–95
Scadding SR, Maden M (1986a) Comparison of the effects of vitamin-a on limb development and regeneration in Xenopus laevis tadpoles. J Embryol Exp Morphol 91:35–53
Scadding SR, Maden M (1986b) The effects of local application of retinoic acid on limb development and regeneration in tadpoles of Xenopus laevis. J Embryol Exp Morphol 91:55–63
Schotthoefer AM, Koehler AV, Meteyer CU, Cole RA (2003) Influence of Ribeiroia ondatrae (Trematoda: Digenea) infection on limb development and survival of northern leopard frogs (Rana pipiens): effects of host stage and parasite-exposure level. Can J Zool 81:1144–1153
Sessions SK, Ruth SB (1990) Explanation for naturally occurring supernumerary limbs in amphibians. J Exp Zool 254:38–47
Sessions SK, Franssen RA, Horner VL (1999) Morphological clues from multilegged frogs: are retinoids to blame? Science 284:800–802
Stopper GF, Hecker L, Franssen RA, Sessions SK (2002) How trematodes cause limb deformities in amphibians. J Exp Zool 294:252–263
Szuroczki D, Richardson JML (2009) The role of trematode parasites in larval anuran communities: an aquatic ecologist's guide to the major players. Oecologia 161:371–385
Thomas F, Adamo S, Moore J (2005) Parasitic manipulation: where are we and where should we go? Behav Process 68:185–199
Acknowledgements
We would especially like to thank Alysen Lougheed for all of her help in the seemingly never ending snail hunt and preparation of samples and Amanda Lepp for her assistance in the earlier experimental design phase. We would also like thank Dr. Janet Koprivnikar for all of her valuable input on earlier drafts of the manuscript. This research was funded by the Department of Biological Sciences, Brock University and the Natural Sciences and Engineering Research Council (DG# 238373 to G.E.S and DG# 2802-07 to R.L.C).
Author information
Authors and Affiliations
Corresponding author
Additional information
D. Szuroczki and N. D. Vesprini shared primary authorship as both parties contributed equally to every aspect of this research.
Rights and permissions
About this article
Cite this article
Szuroczki, D., Vesprini, N.D., Jones, T.R.B. et al. Presence of Ribeiroia ondatrae in the developing anuran limb disrupts retinoic acid levels. Parasitol Res 110, 49–59 (2012). https://doi.org/10.1007/s00436-011-2451-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-011-2451-z