Abstract
Background and aims
The clinical value of synbiotics in surgical patients remains unclear. The aim of this study was to investigate the effect of synbiotics on intestinal integrity and microflora, as well as on surgical outcome, in patients undergoing high-risk hepatectomy.
Methods
Fifty-four patients with biliary cancer were randomly allocated to two groups before hepatectomy. One group received postoperative enteral feeding that included synbiotics; the other received enteral feeding only. Lactulose/mannitol (L/M) ratio, serum diamine oxidase (DAO) activity, and fecal microflora and organic acid concentrations were determined. Postoperative infectious complications were recorded.
Results
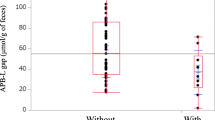
Of the 54 patients, 44 completed the trial (21 receiving synbiotics and 23 others as controls). Postoperative changes in L/M ratios and serum DAO activities were identical between the two groups. Numbers of beneficial bacteria increased in the synbiotics group after surgery but decreased in controls. Numbers of harmful microorganisms decreased in the synbiotics group but increased in controls. Total organic acid concentrations increased in the synbiotics group but decreased in controls. Incidence of infectious complications was 19% (4/21) in the synbiotics group and 52% (12/23) in controls (P<0.05). All study patients tolerated surgery (mortality 0%).
Conclusions
Synbiotics, combined with early enteral nutrition, can reduce postoperative infections. This beneficial effect presumably involves correction of an intestinal microbial imbalance induced by surgical stress.



Similar content being viewed by others
References
Nagino M, Nimura Y, Hayakawa N, Kamiya J, Kondo S, Sasaki R, Hamajima N (1993) Logistic regression and discriminant analyses of hepatic failure after liver resection for carcinoma of the biliary tract. World J Surg 17:250–255
Pichlmayr R, Weimann A, Klempnauer J, Oldhafer KJ, Maschek H, Tusch G, Ringe B (1996) Surgical treatment in proximal bile duct cancer. A single-center experience. Ann Surg 224:628–638
Nimura Y, Kamiya J, Nagino M, Kanai M, Uesaka K, Kondo S, Hayakawa N (1998) Aggressive surgical treatment of hilar cholangiocarcinoma. J Hepatobiliary Pancreat Surg 5:52–61
Neuhaus P, Jonas S, Bechstein WO, Lohmann R, Radke C, Kling N, Wex C, Lobeck H, Hintze R (1999) Extended resection for hilar cholangiocarcinoma. Ann Surg 230:808–819
Nagino M, Kamiya J, Uesaka K, Sano T, Yamamoto H, Hayakawa N, Kanai M, Nimura Y (2001) Complications of hepatectomy for hilar cholangiocarcinoma. World J Surg 27:1277–1283
Sitzmann JV, Greene PS (1994) Perioperative predictors of morbidity following hepatic resection for neoplasms. Ann Surg 219:13–17
Shigeta H, Nagino M, Kamiya J, Uesaka K, Sano T, Yamamoto H, Hayakawa N, Kanai M, Nimura Y (2002) Bacteremia after hepatectomy: an analysis of single center, 10-year experience with 407 patients. Langenbecks Arch Surg 387:117–124
Wang X, Soltesz V, Andersson R, Bengmark S (1993) Bacterial translocation in acute liver failure induced by 90 per cent hepatectomy in the rat. Br J Surg 80:66–71
Tancrede CH, Andremont AO (1985) Bacterial translocation and gram-negative bacteremia in patients with hematological malignancies. J Infect Dis 152:99–103
Lilly D, Stillwell RJ (1965) Probiotics: growth promoting factors produced by microorganisms. Science 147:747–748
Fuller R (1991) Probiotics in human medicine. Gut 32:439–442
Gibson GR, Beatty ER, Wang X, Cummings JH (1995) Selective stimulation of Bifidobacteria in the human colon by oligofructose and inulin. Gastroenterology 108:975–982
Colins MD, Gibson GR (1999) Probiotics, prebiotics, and synbiotics: approaches for modulating the microbial ecology of the gut. Am J Clin Nutr 69 [Suppl]:1052S–1057S
McNaught CE, Woodcock NP, MacFie J (2002) A prospective randomized study of the probiotic Lactobacillus plantarum 299V on indices of gut barrier function in elective surgical patients. Gut 51:827–831
Rayes N, Seehofer D, Hansen S, Boucsein K, Muller AR, Serke S, Bengmark S, Neuhaus P (2002) Early enteral supply of lactobacillus and fiber versus selective bowel decontamination: a controlled trial in liver transplant recipients. Transplantation 74:123–128
Rayes N, Hansen S, Seehofer D, Muller AR, Serke S, Bengmark S (2002) Early enteral supply of fiber and Lactobacilli versus conventional nutrition: a controlled trial of patients with major abdominal surgery. Nutrition 18:609–615
Kamiya S, Nagino M, Kanazawa H, Komatsu S, Mayumi T, Takagi K, Asahara T, Nomoto K, Tanaka R, Nimura Y (2004) The value of bile replacement during external biliary drainage: an analysis of intestinal permeability, integrity, and microflora. Ann Surg 239:510–517
Jansen G, Muskiet FA, Schierbeek H, Berger R, van der Slik W (1986) Capillary gas chromatographic profiling of urinary, plasma and erythrocyte sugars and polyols as their trimethylsilyl derivatives, preceded by a simple and rapid prepurification method. Clin Chim Acta 157:277–293
Takagi K, Nakao M, Ogura Y, Nabeshima T, Kunii A (1994) Sensitive colorimetric assay of serum diamine oxidase. Clin Chim Acta 226:67–75
Azuma R, Suto T (1970) Validity of transfer of the taxonomical position of Corynebacterium pseudopyogenes from genus Corynebacterium to genus Actinomyces. In: Izuka H, Hasegawa T (eds) Proceeding of the first international conference on culture collection. University of Tokyo Press, Tokyo, pp 493–505
Tanaka R, Mutai M (1980) Improved medium for selective isolation and enumeration of Bifidobacterium. Appl Environ Microbiol 40:866–869
Petts DN (1984) Colistin–oxolinic acid–blood agar: a new selective medium for streptococci. J Clin Microbiol 19:4–7
Sonoike K, Mada M, Mutai M (1986) Selective agar medium for counting viable cells of bifidobacteria in fermented milk (in Japanese with English abstract). J Food Hyg Soc Jpn 27:238–244
Kitajima H, Sumida Y, Tanaka R, Yuki N, Takayama H, Fujimura M (1997) Early administration of Bifidobacterium breve to preterm infants: randomized controlled trial. Arch Dis Child 76:101–107
Kikuchi H, Yajima T (1992) Correlation between water-holding capacity of different types of cellulose in vitro and gastrointestinal retention time in vivo of rats. J Sci Food Agric 60:139–146
Mochizuki H, Trocki O, Dominioni L, Brackett KA, Joffe SN, Alexander JW (1984) Mechanism of prevention of postburn hypermetabolism and catabolism by early enteral feeding. Ann Surg 200:297–310
Gionotti L, Alexander JW, Nelson JL (1994) Role of early enteral feeding and acute starvation on postburn bacterial translocation and host defense: prospective, randomized trials. Crit Care Med 22:265–272
Kudsk KA, Croce MA, Fabian TC, Minard G, Tolley EA, Poret HA (1992) Enteral versus parenteral feeding: effects on septic morbidity after blunt and penetrating abdominal trauma. Ann Surg 215:503–513
Omura K, Hirano K, Kanehira E, Kaito K, Tamura M, Nishida S, Kawakami K, Watanabe Y (2000) Small amount of low-residue diet with parenteral nutrition can prevent decreases in intestinal mucosal integrity. Ann Surg 231:112–118
Yuki N, Watanabe K, Mike A, Tagami Y, Tanaka R, Ohwaki M, Morotomi M (1999) Survival of a probiotic, Lactobacillus casei Shirota, in the gastrointestinal tract: selective isolation from feces and identification using monoclonal antibodies. Int J Food Microbiol 48:51–57
Welsh FK, Ramsden CW, MacLennan K, Sheridan MB, Barclay GR, Guillou PJ, Reynolds JV (1998) Increased intestinal permeability and altered mucosal immunity in cholestatic jaundice. Ann Surg 227:205–212
Luk G, Bayless TM, Baylin SB (1983) Plasma postheparin diamine oxidase. J Clin Invest 71:1308–1315
Mitsuoka T (2000) Significance of dietary modulation of intestinal flora and intestinal environment. Biosci Microflora 19:15–25
Schneider SM, Le Gall P, Girard-Pipau F, Piche T, Pompei A, Nano JL, Hebuterne X, Rampal P (2000) Total artificial nutrition is associated with major changes in the fecal flora. Eur J Nutr 39:248–255
Guerrant GO, Lambert MA, Moss CW (1982) Analysis of short-chain acids from anaerobic bacteria by high-performance liquid chromatography. J Clin Microbiol 16:355–360
Takagi A, Matsuzaki T, Sato M, Nomoto K, Morotomi M, Yokokura T (1999) Inhibitory effect of oral administration of Lactobacillus casei on 3-methylcholanthrene-induced carcinogenesis in mice. Med Microbiol Immunol 188:111–116
Yasui H, Nagaoka N, Hayakawa K (1994) Augmentation of anti-influenza virus hemagglutinin antibody production by Peyer’s patch cells with Bifidobacterium breve YIT4064. Clin Diagn Lab Immunol 1:244–246
Kato I, Tanaka K, Yokokura T (1999) Lactic acid bacterium potently induces the production of interleukin-12 and interferon-gamma by mouse splenocytes. Int J Immunopharmacol 21:121–131
Yasui H, Nagaoka N, Mike A (1991) Enhancement of immune response in Peyer’s patch cells cultured with Bifidobacterium breve. J Dairy Sci 74:1187–1195
Takagi A, Matsuzaki T, Sato M, Nomoto K, Morotomi M, Yokokura T (2001) Enhancement of natural killer cytotoxicity delayed murine carcinogenesis by a probiotic microorganism. Carcinogenesis 22:599–605
Sakata T (1995) Effect of short-chain fatty acids on the proliferation of gut epithelial cells in vivo. In: Cummings JH, Rombeau JL, Sakata T (eds) Physiological and clinical aspects of short-chain fatty acids. Cambridge University Press, Cambridge, pp 289–305
Cherbut C (1995) Effects of short-chain fatty acids on gastrointestinal motility. In: Cummings JH, Rombeau JL, Sakata T (ed) Physiological and clinical aspects of short-chain fatty acids. Cambridge University Press, Cambridge, pp 191–208
Willemsen LE, Koetsier MA, van Deventer SJH, van Tol EA (2003) Short chain fatty acids stimulate epithelial mucin2 expression through differential effects on prostaglandin E1 and E2 production by intestinal myofibroblasts. Gut 52:1442–1447
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kanazawa, H., Nagino, M., Kamiya, S. et al. Synbiotics reduce postoperative infectious complications: a randomized controlled trial in biliary cancer patients undergoing hepatectomy. Langenbecks Arch Surg 390, 104–113 (2005). https://doi.org/10.1007/s00423-004-0536-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-004-0536-1