Abstract
Purpose
Exercise training improves endothelium-dependent vasodilation, whereas hypoxic stress causes vascular endothelial dysfunction. Monocyte-derived endothelial progenitor cells (Mon-EPCs) contribute to vascular repair process by differentiating into endothelial cells. This study investigates how high-intensity interval (HIT) and moderate-intensity continuous (MCT) exercise training affect circulating Mon-EPC levels and EPC functionality under hypoxic condition.
Methods
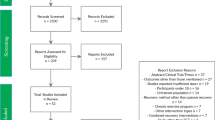
Sixty healthy sedentary males were randomized to engage in either HIT (3-min intervals at 40 and 80 % VO2max for five repetitions, n = 20) or MCT (sustained 60 % VO2max, n = 20) for 30 min/day, 5 days/week for 6 weeks, or to a control group (CTL) that did not received exercise intervention (n = 20). Mon-EPC characteristics and EPC functionality under hypoxic exercise (HE, 100 W under 12 % O2) were determined before and after HIT, MCT, and CTL.
Results
The results demonstrated that after the intervention, the HIT group exhibited larger improvements in VO2peak, estimated peak cardiac output (QC), and estimated peak perfusions of frontal cerebral lobe (QFC) and vastus lateralis (QVL) than the MCT group. Furthermore, HIT (a) increased circulating CD14++/CD16−/CD34+/KDR+ (Mon-1 EPC) and CD14++/CD16+/CD34+/KDR+ (Mon-2 EPC) cell counts, (b) promoted the migration and tube formation of EPCs, (c) diminished the shedding of endothelial (CD34−/KDR+/phosphatidylserine+) cells, and (d) elevated plasma nitrite plus nitrate, stromal cell-derived factor-1, matrix metalloproteinase-9, and vascular endothelial growth factor-A concentrations at rest or following HE, compared to those of MCT. In addition, Mon-1 and -2 EPC counts were directly related to VO2peak and estimated peak QC, QFC, and QVL.
Conclusions
HIT is superior to MCT for improving hemodynamic adaptation and Mon-EPC production. Moreover, HIT effectively enhances EPC functionality and suppresses endothelial injury undergoing hypoxia.


Similar content being viewed by others
Abbreviations
- Mon-EPCs:
-
Monocyte-derived endothelial progenitor cells
- HIT:
-
High-intensity interval
- MCT:
-
Moderate-intensity continuous
- HE:
-
Hypoxic exercise
- QC :
-
Cardiac output
- QFC :
-
Peak perfusions of frontal cerebral lobe
- QVL :
-
Vastus lateralis
- Mon-1 EPC:
-
CD14++/CD16−/CD34+/KDR+
- Mon-2 EPC:
-
CD14++/CD16+/CD34+/KDR+
- KDR+ :
-
Kinase domain receptor-positive
- CTL:
-
Control group
- VO2max :
-
Maximal O2 consumption
- HR:
-
Heart rate
- GXT:
-
Graded exercise test
- V E :
-
Minute ventilation
- VCO2 :
-
Carbonic dioxide production
- NICOM:
-
Noninvasive continuous cardiac output monitoring system
- SV:
-
Stroke volume
- SVC:
-
Systemic vascular conductance
- NIR:
-
Near-infrared
- FC:
-
Left frontal cortex
- VL:
-
Left vastus lateralis muscle
- PBMCs:
-
Peripheral blood mononuclear cells
- FITC:
-
Fluorescein isothiocyanate
- PE:
-
Phycoerythrin
- HSC:
-
Hemangioblast stem cell
- EPC:
-
Early endothelial progenitor cell
- CEP:
-
Endothelial precursor cell
- PS+ :
-
CD34−/KDR+/phosphatidylserine+
- ECIS:
-
Electric cell-substrate impedance sensing
- ET:
-
Effective time
- T max :
-
Maximum impedance
- NO:
-
Nitric oxide
References
Aicher A et al (2003) Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat Med 9:1370–1376
American college of sports medicine (2014) Clinical exercise testing. In: ACSM’s Guidelines for exercise testing and prescription. Lippincott Williams & Wilkins, Philadelphia, 9th edn. p 114–141
Bruno S, Bussolati B, Scacciatella P, Marra S, Sanavio F, Tarella C, Camussi G (2006) Combined administration of G-CSF and GM-CSF stimulates monocyte-derived pro-angiogenic cells in patients with acute myocardial infarction. Cytokine 34:56–65
Buono MJ, Miller PW, Hom C, Pozos RS, Kolkhorst FW (2005) Skin blood flow affects in vivo near-infrared spectroscopy measurements in human skeletal muscle. Jpn J Physiol 55:241–244
Byrne AM, Bouchier-Hayes DJ, Harmey JH (2005) Angiogenic and cell survival functions of vascular endothelial growth factor (VEGF). J Cell Mol Med 9:777–794
Chen YC, Ho CW, Tsai HH, Wang JS (2015) Interval and continuous exercise regimens suppress neutrophil-derived microparticle formation and neutrophil-promoted thrombin generation under hypoxic stress. Clin Sci (Lond) 128:425–436
Crimi E, Ignarro LJ, Cacciatore F, Napoli C (2009) Mechanisms by which exercise training benefits patients with heart failure. Nat Rev Cardiol 6:292–300
Dimmeler S (2010) Regulation of bone marrow-derived vascular progenitor cell mobilization and maintenance. Arterioscler Thromb Vasc Biol 30:1088–1093
Dresske B et al (2006) Multipotent cells of monocytic origin improve damaged heart function. Am J Transplant 6:947–958
Duncan A, Meek JH, Clemence M, Elwell CE, Tyszczuk L, Cope M, Delpy DT (1995) Optical pathlength measurements on adult head, calf and forearm and the head of the newborn infant using phase resolved optical spectroscopy. Phys Med Biol 40:295–304
Esaki K et al (2005) Association between regional quadriceps oxygenation and blood oxygen saturation during normoxic one-legged dynamic knee extension. Eur J Appl Physiol 95:361–370
Ferrari M, Muthalib M, Quaresima V (2011) The use of near-infrared spectroscopy in understanding skeletal muscle physiology: recent developments. Philos Trans A Math Phys Eng Sci 369:4577–4590
Fu TC et al (2013) Aerobic interval training improves oxygen uptake efficiency by enhancing cerebral and muscular hemodynamics in patients with heart failure. Int J Cardiol 167:41–50
Fujiyama S et al (2003) Bone marrow monocyte lineage cells adhere on injured endothelium in a monocyte chemoattractant protein-1-dependent manner and accelerate reendothelialization as endothelial progenitor cells. Circ Res 93:980–989
Gielen S, Schuler G, Adams V (2010) Cardiovascular effects of exercise training: molecular mechanisms. Circulation 122:1221–1238
Grassi B et al (2003) Muscle oxygenation and pulmonary gas exchange kinetics during cycling exercise on-transitions in humans. J Appl Physiol 95:149–158
Harraz M, Jiao C, Hanlon HD, Hartley RS, Schatteman GC (2001) CD34− blood-derived human endothelial cell progenitors. Stem Cells 19:304–312
Heissig B et al (2002) Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell 109:625–637
Henson LC, Calalang C, Temp JA, Ward DS (1998) Accuracy of a cerebral oximeter in healthy volunteers under conditions of isocapnic hypoxia. Anesthesiology 88:58–65
Hsu CC, Tsai WC, Chen CP, Lu YM, Wang JS (2010) Effects of negative pressures on epithelial tight junctions and migration in wound healing. Am J Physiol Cell Physiol 299:C528–C534
Huang SC et al (2014) Modified high-intensity interval training increases peak cardiac power output in patients with heart failure. Eur J Appl Physiol 114:1853–1862
Kanki S et al (2011) Stromal cell-derived factor-1 retention and cardioprotection for ischemic myocardium. Circ Heart Fail 4:509–518
Kubo H, Berretta RM, Jaleel N, Angert D, Houser SR (2009) c-Kit + bone marrow stem cells differentiate into functional cardiac myocytes. Clin Transl Sci 2:26–32
Laufs U et al (2004) Physical training increases endothelial progenitor cells, inhibits neointima formation, and enhances angiogenesis. Circulation 109:220–226
Lee PH, Macfarlane DJ, Lam TH, Stewart SM (2011) Validity of the international physical activity questionnaire short form (IPAQ-SF): a systematic review. Int J Behav Nutr Phys Act 8:115
Lenk K, Uhlemann M, Schuler G, Adams V (2011) Role of endothelial progenitor cells in the beneficial effects of physical exercise on atherosclerosis and coronary artery disease. J Appl Physiol 111:321–328
Murasawa S, Asahara T (2005) Endothelial progenitor cells for vasculogenesis. Physiology (Bethesda) 20:36–42
Ortega RM, Pérez-Rodrigo C, López-Sobaler AM (2015) Dietary assessment methods: dietary records. Nutr Hosp 31:S38–S45
Ramos JS, Dalleck LC, Tjonna AE, Beetham KS, Coombes JS (2015) The impact of high-intensity interval training versus moderate-intensity continuous training on vascular function: a systematic review and meta-analysis. Sports Med 45:679–692
Rohde E et al (2006) Blood monocytes mimic endothelial progenitor cells. Stem Cells 24:357–367
Schmeisser A et al (2001) Monocytes coexpress endothelial and macrophagocytic lineage markers and form cord-like structures in Matrigel under angiogenic conditions. Cardiovasc Res 49:671–680
Shantsila E, Wrigley B, Tapp L, Apostolakis S, Montoro-Garcia S, Drayson MT, Lip GY (2011) Immunophenotypic characterization of human monocyte subsets: possible implications for cardiovascular disease pathophysiology. J Thromb Haemost 9:1056–1066
Shantsila E, Wrigley BJ, Shantsila A, Tapp LD, Gill PS, Lip GY (2012) Monocyte-derived and CD34+/KDR+ endothelial progenitor cells in heart failure. J Thromb Haemost 10:1252–1261
Subudhi AW, Dimmen AC, Roach RC (2007) Effects of acute hypoxia on cerebral and muscle oxygenation during incremental exercise. J Appl Physiol 103:177–183
Swain DP, Leutholtz BC, King ME, Haas LA, Branch JD (1988) Relationship between % heart rate reserve and % VO2 reserve in treadmill exercise. Med Sci Sports Exerc 30:318–321
Tjonna AE et al (2008) Aerobic interval training versus continuous moderate exercise as a treatment for the metabolic syndrome: a pilot study. Circulation 118:346–354
Tran TK et al (1999) Comparative analysis of NMR and NIRS measurements of intracellular PO2 in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol 276:R1682–R1690
Van Beekvelt MC, Colier WN, Wevers RA, Van Engelen BG (2001) Performance of near-infrared spectroscopy in measuring local O2 consumption and blood flow in skeletal muscle. J Appl Physiol 90:511–519
van der Zee P et al (1992) Experimentally measured optical pathlengths for the adult head, calf and forearm and the head of the newborn infant as a function of inter optode spacing. Adv Exp Med Biol 316:143–153
Wang JS, Chen LY, Fu LL, Chen ML, Wong MK (2007) Effects of moderate and severe intermittent hypoxia on vascular endothelial function and haemodynamic control in sedentary men. Eur J Appl Physiol 100:127–135
Wang JS, Cheng ML, Yen HC, Lou BS, Liu HC (2009) Vitamin E suppresses enhancement of factor VIII-dependent thrombin generation by systemic hypoxia. Stroke 40:656–659
Wang JS et al (2013) Effect of aerobic interval training on erythrocyte rheological and hemodynamic functions in heart failure patients with anemia. Int J Cardiol 168:1243–1250
Wang JS, Lee MY, Lien HY, Weng TP (2014) Hypoxic exercise training improves cardiac/muscular hemodynamics and is associated with modulated circulating progenitor cells in sedentary men. Int J Cardiol 170:315–323
Weng TP, Huang SC, Chuang YF, Wang JS (2013) Effects of interval and continuous exercise training on CD4 lymphocyte apoptotic and autophagic responses to hypoxic stress in sedentary men. PLoS ONE 8:e80248
Whaley MH, Brubaker PH, Kaminsky LA, Miller CR (1997) Validity of rating of perceived exertion during graded exercise testing in apparently healthy adults and cardiac patients. J Cardiopulm Rehabil 17:167–261
Wisloff U et al (2007) Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation 115:3086–3094
Yellon DM, Downey JM (2003) Preconditioning the myocardium: from cellular physiology to clinical cardiology. Physiol Rev 83:1113–1151
Zampetaki A, Kirton JP, Xu Q (2008) Vascular repair by endothelial progenitor cells. Cardiovasc Res 78:413–421
Acknowledgments
The authors would like to thank the volunteers for their enthusiastic participation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by the National Science Council of Taiwan (Grant Number NSC 100-2314-B-182-004-MY3), Chang Gung Medical Research Program (Grant number CMRPD190173), and Healthy Aging Research Center, Chang Gung University (Grant Number EMRPD1A0841).
Conflict of interest
No conflicts of interest, financial or otherwise, are declared by the authors.
Additional information
Communicated by David C. Poole.
Rights and permissions
About this article
Cite this article
Tsai, HH., Lin, CP., Lin, YH. et al. High-intensity Interval training enhances mobilization/functionality of endothelial progenitor cells and depressed shedding of vascular endothelial cells undergoing hypoxia. Eur J Appl Physiol 116, 2375–2388 (2016). https://doi.org/10.1007/s00421-016-3490-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-016-3490-z