Abstract
Purpose
Anti-VEGF treatment is the therapy of choice in age-related macular degeneration and is also applied in diabetic macular edema or retinal vein occlusion. Recently, aflibercept has been approved for therapeutic use. In this study, we investigate the efficacy of aflibercept in comparison with the VEGF-antagonists ranibizumab and bevacizumab in RPE/choroid organ cultures.
Methods
RPE/choroid organ cultures were prepared from freshly slaughtered pigs’ eyes. Organ cultures were treated with 125 μg/ml aflibercept, ranibizumab, or bevacizumab, and the VEGF content of the supernatant was evaluated over the course of 7 days. Additionally, the minimal concentration of VEGF inhibition was evaluated in organ cultures, measured after 6 h of application.
Results
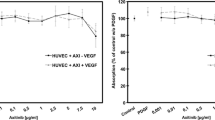
Aflibercept was able to completely inhibit VEGF detection for 6 h at a minimal concentration of 0.031 μg/ml, in contrast to bevacizumab (3.9 μg/ml) and ranibizumab (0.244 μg/ml). A statistically significant VEGF inhibition compared to control could be found for aflibercept and ranibizumab down to and including 0.031 μg/ml, while bevacizumab was significantly reduced compared to control down to a concentration of 0.244 μg/ml and again at 0.061 μg/ml. Inhibition of VEGF after a single aflibercept application of 125 μg/ml could be found over the course of 7 days, with some VEGF detectable at the 7th day. In contrast, VEGF was detectable after 72 h of ranibizumab treatment and some VEGF could already be found 12 h after bevacizumab treatment.
Conclusions
In conclusion, aflibercept displays a prolonged VEGF inhibition, confirming its effectiveness but also raising concerns about possible side effects of long-term usage.



Similar content being viewed by others
References
Velez-Montoya R, Oliver SCN, Olson JL, Fine SL, Mandava N, Quiroz-Mercado H (2013) Current knowledge and trends in age-related macular degeneration. Today’s and futures treatments. Retina 33:1487–1502
Mitchell P, Korobelnik JF, Lanzetta P et al (2010) Ranibizumab (Lucentis) in neovascular age-related macular degeneration: evidence from clinical trials. Br J Ophthalmol 94:2–13
Heier JS, Brown DM, Chong V et al (2012) Intravitreal aflibercept (VEGF Trap-Eye) in wet age-related macular degeneration. Ophthalmology 119:2537–2548
Jyothi S, Chowdhury H, Elagouz M, Sivaprasad S (2010) Intravitreal bevacizumab (Avastin) for age-related macular degeneration: a critical analysis of literature. Eye 24:816–824
Harding SP (2010) Neovascular age-related macular degeneration: decision-making and optimal management. Eye 24:497–505
Hörster R, Ristau T, Sadda SR, Liakopoulos S (2011) Individual recurrence intervals after anti-VEGF therapy for age-related macular degeneration Graefes Arch Clin Exp Ophthalmol 249:645–652
Klettner A (2013) Physiological functions of VEGF in the retina and its possible implications on prolonged VEGF therapy. In: Parker ML (ed) Vascular Endothelial Growth Factor (VEGF), Biology, Regulation and Clinical Significance. Nova Biomedical, New York, pp 117–136
Peters S, Heiduschka P, Julien S, Ziemssen F, Fietz H, Bartz-Schmidt KU, The Tübingen Bevacizumab Study Group, Schraermeyer U (2007) Ultrastructural findings in the primate eye after intravitreal injection of bevacizumab. Am J Ophthalmol 143:995–1002
Gerber HP, McMurtrey A, Kowalski J et al (1998) Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3’kinase/Akt signal transduction pathway. J Biol Chem 273:30336–30343
Byeon S, Lee S, Choi S et al (2010) Vascular endothelial growth factor as an autocrine survival factor for retinal pigment epithelial cells under oxidative stress via the VEGF-R2/PI3K/Akt. Invest Ophthalmol Vis Sci 51:1190–1197
Kilic Ü, Kilic E, Järve A et al (2006) Human vascular endothelial growth factor protects axotomized retinal ganglion cells in vivo by activating Erk1/2 and Akt pathways. J Neurosci 26:12439–12446
Ueno S, Pease ME, Wersinger DM et al (2008) Prolonged blockade of VEGF family members does not cause identifiable damage to retinal neurons or vessels. J Cell Physiol 217:13–22
Miki A, Miki K, Ueno S et al (2010) Prolonged blockade of VEGF receptors does not damage retinal photoreceptors or ganglion cells. J Cell Physiol 224:262–272
Kurihara T, Westenskow PD, Bravo S, Aguilar E, Friedlander M (2010) Targeted deletion of Vegfa in adult mice induces vision loss. J Clin Invest 122:4213–4217
Ford KM, Saint-Geniez M, Walshe T, Zahr A, D’Amore PA (2011) Expression and role of VEGF in the adult retinal pigment epithelium. Invest Ophthalmol Vis 52:9478–9487
Saint-Geniez M, Maharaj ASR, Walshe TE et al (2008) Endogenous VEGF is required for visual function: evidence for a survival role on Müller cells and photoreceptors. PloS One 3:1–13
Rosenfeld PJ, Shapiro H, Tuomi L, Webster M, Elledge J, Blodi B; MARINA and ANCHOR Study Groups (2011) Characteristics of patients losing vision after 2 years of monthly dosing in the phase III ranibizumab clinical trials. Ophthalmology 118:523–530
Mariani A, Deli A, Ambresin A, Mantel I (2011) Characteristics of eyes with secondary loss of visual acuity receiving variable dosing ranibizumab for neovascular age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol 249:1635–1642
Klettner A, Roider J (2008) Comparison of bevacizumab, ranibizumab, and pegaptanib in vitro: efficiency and possible additional pathways. Invest Ophthalmol Vis Sci 49:4523–4527
Klettner A, Westhues D, Lassen J, Bartsch S, Roider J (2013) Regulation of constitutive vascular endothelial growth factor secretion in retinal pigment epithelium/choroid organ cultures: p38, nuclear factor kappaB, and the vascular endothelial growth factor receptor-2/phosphatidylinositol 3 kinase pathway. Mol Vis 19:281–291
Minuth WW, Kloth S, Aigner J, Sittinger M, Röckl W (1996) Approach to an organo-typical environment for cultured cells and tissues. Biotechniques 20:498–501
Miura Y, Klettner A, Noelle B, Hasselbach H, Roider J (2010) Change of morphological and functional characteristics of retinal pigment epithelium cells during cultivation of retinal pigment epithelium–choroid perfusion tissue culture. Ophthalmic Res 43:122–133
Lassota N (2008) Clinical and histological aspects of CNV formation: studies in an animal model. Acta Ophthalmol 86(2):1–24
Sanchez I, Martin R, Ussa F, Fernandez-Bueno I (2011) The parameters of the porcine eyeball. Graefes Arch Clin Exp Ophthalmol 249:475–482
Middleton S (2010) Porcine ophthalmology. Vet Clin Food Anim 26:557–572
Butler J, Sun J, Wertz N, Sinkora M (2006) Antibody repertoire development in swine. Dev Comp Immunol 30:199–211
Yang P, Chen L, Zwart R, Kijlstra A (2002) Immune cells in the porcine retina: distribution, characterization and morphological features. Invest Ophthalmol Vis Sci 43:1488–1492
Klettner A, Koinzer S, Meyer T, Roider J (2013) Toll-like receptor 3 activation in retinal epithelium cells—mitogen-activated protein kinase pathways of cell death and vascular endothelial growth factor secretion. Acta Ophthalmol 91:e211–218
Yu L, Liang XH, Ferrara N (2011) Comparing protein VEGF-inhibitors: invitro biological studies. Biochem Biophys Res Commun 408:276–281
Papadopoulos N, Martin J, Ruan Q etal (2012) Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF trap, ranibizumab, bevacizumab. Angiogenesis 15:171–185
Shiose S, Hata Y, Noda Y, Sassa Y, Takeda A, Yoshikawa H, Fujisawa K, Kubota T, Ishibashi T (2004) Fibrinogen stimulates invitro angiogenesis by choroidal endothelial cells via autocrine VEGF. Graefes Arch Clin Exp Ophthalmol 242:777–83
Jin J, Yuan F, Shen MQ, Feng YF, He QL (2013) Vascular endothelial growth factor regulates primate choroid-retinal endothelial cell proliferation and tube formation through PI3K/Akt and MEK/ERK dependent signaling. Mol Cell Biochem 381:267–72
Ottino P, Finley J, Rojo E, Ottlecz A, Lambrou GN, Bazan HE, Bazan NG (2004) Hypoxia activates matrix metalloproteinase expression and the VEGF system in monkey choroid-retinal endothelial cells: involvement of cytosolic phospholipase A2 activity. Mol Vis 10:341–50
Klettner A, Doths J, Roider J (2012) Nicotine reduces VEGF-secretion and phagocytotic activity in porcine RPE. Graefes Arch Clin Exp Ophthalmol 250:33–38
Acknowledgments
This research was financially supported by a Novartis research grant. AK has been a consultant for and received lecture fees and travel grants by Novartis Pharma. Parts of the data presented here have been presented at the DOG meeting 2013.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Klettner, A., Recber, M. & Roider, J. Comparison of the efficacy of aflibercept, ranibizumab, and bevacizumab in an RPE/choroid organ culture. Graefes Arch Clin Exp Ophthalmol 252, 1593–1598 (2014). https://doi.org/10.1007/s00417-014-2719-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-014-2719-y