Abstract
Objective
No consensus exists on the number of chemotherapy cycles to be administered before and after interval debulking surgery (IDS) in patients with advanced stage epithelial ovarian cancer. The present study aims to explore the optimal number of cycles of neoadjuvant chemotherapy (NAC) and post-operation chemotherapy to treat the International Federation of Gynecology and Obstetrics stage IIIc–IV high-grade serous ovarian cancer (HG-SOC).
Materials and Methods
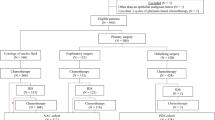
A total of 129 IIIc–IV stage HG-SOC cases were retrospectively analyzed. Cases were comprised of patients who underwent NAC followed by IDS and who achieved clinical complete response (CCR) at the end of primary therapy. Patients were recruited from the Jiangsu Institute of Cancer Research between 1993 and 2013. Optimal IDS-associated factors were explored with logistic regression. The association between progression-free survival (PFS), overall survival (OS) duration, and covariates was assessed by Cox proportional hazards model and log-rank test.
Results
The median number of NAC cycle was 3 (range 1–8). CA-125 decreasing kinetics (p = 0.01) was independently associated with optimal IDS. CA-125 decreasing kinetics, optimal IDS, and NAC cycles was independently associated with OS (p < 0.01, p < 0.01, p = 0.03, respectively) and PFS (p < 0.01, p < 0.01, p = 0.04, respectively). The PFS of patients who underwent ≥5 NAC cycles was shorter than those of patients who underwent <5 NAC cycles (12.3 versus 17.2 months). The PFS and OS of patients who underwent <5 cycles of adjuvant chemotherapy post-IDS were shorter than those of patients who underwent ≥5 cycles (14.2 and 20.3 versus 21.2 and 28.8 months).
Conclusion
NAC cycles, CA-125 decreasing kinetics, and optimal debulking are independently associated with the prognosis of patients with advanced stage HG-SOC who underwent NAC/IDS and achieved CCR. The number of administered NAC cycles should not exceed 4.


Similar content being viewed by others
Abbreviations
- IDS:
-
Interval cytoreduction surgery
- PDS:
-
Primary debulking surgery
- NAC:
-
Neoadjuvant chemotherapy
- OS:
-
Overall survival
- PFS:
-
Progression free survival
- EOC:
-
Epithelial ovarian cancer
References
Siegel RL et al (2015) Cancer statistics for Hispanics/Latinos, 2015. CA Cancer J Clin 65(6):457–480
Jung KW et al (2015) Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2012. Cancer Res Treat 47(2):127–141
Chen W (2015) Cancer statistics: updated cancer burden in China. Chin J Cancer Res 27(1):1
Torre LA et al (2015) Global cancer statistics, 2012. CA Cancer J Clin 65(2):87–108
Bast RC Jr, Hennessy B, Mills GB (2009) The biology of ovarian cancer: new opportunities for translation. Nat Rev Cancer 9(6):415–428
Chen W et al (2015) Annual report on status of cancer in China, 2011. Chin J Cancer Res 27(1):2–12
Siegel RL, Miller KD, Jemal A (2015) Cancer statistics, 2015. CA Cancer J Clin 65(1):5–29
Hoskins WJ et al (1994) The effect of diameter of largest residual disease on survival after primary cytoreductive surgery in patients with suboptimal residual epithelial ovarian carcinoma. Am J Obstet Gynecol 170(4):974–979 (discussion 979–980)
Bristow RE et al (2002) Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol 20(5):1248–1259
Elattar A et al (2011) Optimal primary surgical treatment for advanced epithelial ovarian cancer. Cochrane Database Syst Rev 8:CD007565
Vergote I, Amant F, Leunen K (2010) Neoadjuvant chemotherapy in advanced ovarian cancer: what kind of evidence is needed to convince US gynaecological oncologists? Gynecol Oncol 119(1):1–2
Chi DS et al (2006) What is the optimal goal of primary cytoreductive surgery for bulky stage IIIC epithelial ovarian carcinoma (EOC)? Gynecol Oncol 103(2):559–564
Vergote I et al (2010) Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med 363(10):943–953
Bian C et al (2016) Primary debulking surgery vs. neoadjuvant chemotherapy followed by interval debulking surgery for patients with advanced ovarian cancer. Arch Gynecol Obstet 293(1):1–6
Petrillo M et al (2014) Prognostic role and predictors of complete pathologic response to neoadjuvant chemotherapy in primary unresectable ovarian cancer. Am J Obstet Gynecol 211(6):632 e1–e8
da Costa Miranda V et al (2014) Neoadjuvant chemotherapy with six cycles of carboplatin and paclitaxel in advanced ovarian cancer patients unsuitable for primary surgery: safety and effectiveness. Gynecol Oncol 132(2):287–291
Giannopoulos T et al (2006) Clinical outcomes of neoadjuvant chemotherapy and primary debulking surgery in advanced ovarian carcinoma. Eur J Gynaecol Oncol 27(1):25–28
Lee SJ et al (2006) Preliminary results of neoadjuvant chemotherapy with paclitaxel and cisplatin in patients with advanced epithelial ovarian cancer who are inadequate for optimum primary surgery. J Obstet Gynaecol Res 32(1):99–106
Bilici A et al (2010) Neoadjuvant chemotherapy followed by interval cytoreductive surgery in patients with unresectable, advanced stage epithelial ovarian cancer: a single centre experience. Arch Gynecol Obstet 282(4):417–425
Wright AA et al (2016) Neoadjuvant chemotherapy for newly diagnosed, advanced ovarian cancer: society of gynecologic oncology and american society of clinical oncology clinical practice guideline. J Clin Oncol 34(28):3460–3473
Bristow RE, Chi DS (2006) Platinum-based neoadjuvant chemotherapy and interval surgical cytoreduction for advanced ovarian cancer: a meta-analysis. Gynecol Oncol 103(3):1070–1076
Onda T, Yoshikawa H (2011) Neoadjuvant chemotherapy for advanced ovarian cancer: overview of outcomes and unanswered questions. Expert Rev Anticancer Ther 11(7):1053–1067
Taşkın S et al (2013) Neoadjuvant chemotherapy equalizes the optimal cytoreduction rate to primary surgery without improving survival in advanced ovarian cancer. Arch Gynecol Obstet 288(6):1399–1403
Vasudev NS et al (2011) The prognostic and predictive value of CA-125 regression during neoadjuvant chemotherapy for advanced ovarian or primary peritoneal carcinoma. Arch Gynecol Obstet 284(1):221–227
Angioli R et al (2013) Can the preoperative HE4 level predict optimal cytoreduction in patients with advanced ovarian carcinoma? Gynecol Oncol 128(3):579–583
Seifert H et al (2015) Poor performance status (PS) is an indication for an aggressive approach to neoadjuvant chemotherapy in patients with advanced epithelial ovarian cancer (EOC). Gynecol Oncol 139(2):216–220
Vallius T et al (2015) 18 F-FDG-PET/CT can identify histopathological non-responders to platinum-based neoadjuvant chemotherapy in advanced epithelial ovarian cancer. Gynecol Oncol 140(1):29–35
Pölcher M et al (2010) Changes in Ki-67 labeling indices during neoadjuvant chemotherapy for advanced ovarian cancer are associated with survival. Int J Gynecol Cancer 20(4):555–560
Pölcher M et al (2010) Foxp3+ cell infiltration and granzyme B+/Foxp3+ cell ratio are associated with outcome in neoadjuvant chemotherapy-treated ovarian carcinoma. Cancer Immunol Immunother 59(6):909–919
Samar MM et al (2015) Vascular endothelial growth factor expression correlates with serum CA125 and represents a useful tool in prediction of refractoriness to platinum-based chemotherapy and ascites formation in epithelial ovarian cancer. Oncotarget 6(29):28491–28501
Xu X et al (2013) Secondary cytoreduction surgery improves prognosis in platinum-sensitive recurrent ovarian cancer. J Exp Clin Cancer Res 32:61
Chen X et al (2013) Cancer stem cells, epithelial-mesenchymal transition, and drug resistance in high-grade ovarian serous carcinoma. Hum Pathol 44(11):2373–2384
Chen X et al (2013) CA-125 level as a prognostic indicator in type I and type II epithelial ovarian cancer. Int J Gynecol Cancer 23(5):815–822
Xu X et al (2013) Nadir CA-125 level as prognosis indicator of high-grade serous ovarian cancer. J Ovarian Res 6:31
Wang F et al (2013) CA-125-indicated asymptomatic relapse confers survival benefit to ovarian cancer patients who underwent secondary cytoreduction surgery. J Ovarian Res 6(1):14
Therasse P et al (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92(3):205–216
Dai-yuan M et al (2013) A meta-analysis: neoadjuvant chemotherapy versus primary surgery in ovarian carcinoma FIGO stageIII and IV. World J Surg Oncol 11:267
Kehoe S et al (2015) Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet 386(9990):249–257
Fagotti A et al (2016) Phase III randomised clinical trial comparing primary surgery versus neoadjuvant chemotherapy in advanced epithelial ovarian cancer with high tumour load (SCORPION trial): final analysis of peri-operative outcome. Eur J Cancer 59:22–33
Onda T et al (2016) Comparison of treatment invasiveness between upfront debulking surgery versus interval debulking surgery following neoadjuvant chemotherapy for stage III/IV ovarian, tubal, and peritoneal cancers in a phase III randomised trial: Japan Clinical Oncology Group Study JCOG0602. Eur J Cancer 64:22–31
Kang S (2015) Neoadjuvant chemotherapy for ovarian cancer: do we have enough evidence? Lancet 386(9990):223–224
van Meurs HS et al (2013) Which patients benefit most from primary surgery or neoadjuvant chemotherapy in stage IIIC or IV ovarian cancer? An exploratory analysis of the European Organisation for Research and Treatment of Cancer 55971 randomised trial. Eur J Cancer 49(15):3191–3201
Cornelis S et al (2012) Role of neoadjuvant chemotherapy in the management of stage IIIC–IV ovarian cancer: survey results from the members of the European Society of Gynecological Oncology. Int J Gynecol Cancer 22(3):407–416
Vergote I et al (2011) Neoadjuvant chemotherapy is the better treatment option in some patients with stage IIIc to IV ovarian cancer. J Clin Oncol 29(31):4076–4078
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This retrospective study was approved by the institutional review board of Jiangsu Cancer Hospital, Nanjing Medical University, China. The informed consent requirement was waived. The committee’s reference number was Jiangsu Cancer Hospital’s Ethical Committee 2016-219.
Conflict of interest
The authors declare that they have no competing interests.
Funding
This study was supported by grants from the National Natural Science Foundation of China (No. 81472441), Natural Science Foundation of Jiangsu Province, China (No. BK20131439), and six major talent summit (No. 2013-wsn-62).
Rights and permissions
About this article
Cite this article
Xu, X., Deng, F., Lv, M. et al. The number of cycles of neoadjuvant chemotherapy is associated with prognosis of stage IIIc–IV high-grade serous ovarian cancer. Arch Gynecol Obstet 295, 451–458 (2017). https://doi.org/10.1007/s00404-016-4256-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-016-4256-x