Abstract
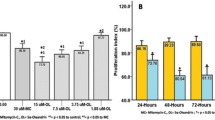
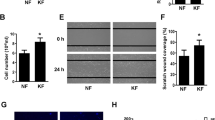
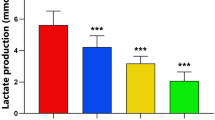
Keloid is a fibrotic disease characterized by abnormal accumulation of extracellular matrix (ECM) in the dermis. It is a late spreading skin overgrowth and may be considered a plastic surgeon’s nightmare. In nature, curcuminoid is composed of curcumin, demethoxycurcumin (DMC) and bisdemethoxycurcumin (bDMC). Curcuminoids have been found to inhibit fibrosis. However, their role in the synthesis of ECM in the keloid fibroblasts (KFs) has remained unclear. In this series of studies, a total of seven primary KFs cultures were used as the KFs model for investigating the inhibitory effect of curcuminoids on the expression of ECM and TGF-β1. A sensitive and reproducible HPLC method was developed to provide a quantitative analysis on the cellular uptake of curcuminoids onto the KF cells. The level of ECM in the primary KFs was elevated. The elevation of ECM and TGF-β1/p-SMAD-2 level was substantially blocked by the cellular uptake of curcumin in a dose-dependent manner in all the seven primary KFs. The results have led to the conclusion that the excessive production of ECM in the KF cells could be blocked and/or rapidly decreased by curcumin.




Similar content being viewed by others
References
Aggarwal BB, Kumar A, Bharti AC (2003) Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res 23:363–398
Ahmad N, Katiyar SK, Mukhtar H (2001) Antioxidants in chemoprevention of skin cancer. Curr Probl Dermatol 29:128–139
Albina JE, Mills CD, Henry WL Jr, Caldwell MD (1990) Temporal expression of different pathway of l-arginine metabolism in healing wounds. J Immunol 144:3877–3880
Bar-Sela G, Epelbaum R, Schaffer M (2010) Curcumin as an anti-cancer agent: review of the gap between basic and clinical applications. Curr Med Chem 17:190–197
España A, Solano T, Quintanilla E (2001) Bleomycin in the treatment of keloids and hypertrophic scars by multiple needle punctures. Dermatol Surg 27:23–27
Hsu YC, Hsiao M, Wang LF, Chien YW, Lee WR (2006) Nitric oxide produced by iNOS is associated with collagen synthesis in keloid scar formation. Nitric Oxide 14:327–334
Hsu YC, Hsiao M, Chien YW, Lee WR (2007) Exogenous nitric oxide stimulated collagen type I expression and TGF-β1 production in keloid fibroblasts by a cGMP-dependent manner. Nitric Oxide 16:258–265
Hsu YC, Wang LF, Chien YW, Lee WR (2007) Induction of TIMP-1 and HSP47 synthesis in primary keloid fibroblasts by exogenous nitric oxide. J Dermatol Sci 45:37–44
Hsu YC, Weng HC, Lin S, Chien YW (2007) Curcuminoids—cellular uptake by human primary colon cancer cells as quantitated by a sensitive HPLC assay and its relation with the inhibition of proliferation and apoptosis. J Agric Food Chem 55:8213–8222
Hu Y, Liang H, Du Y, Zhu Y, Wang X (2010) Curcumin inhibits transforming growth factor-beta activity via inhibition of Smad signaling in HK-2 cells. Am J Nephrol 31:332–341
Kwak HJ, Park MJ, Cho H, Park CM, Moon SI, Lee HC, Park IC, Kim MS, Rhee CH, Hong SI (2006) Transforming growth factor-beta1 induces tissue inhibitor of metalloproteinase-1 expression via activation of extracellular signal-regulated kinase and Sp1 in human fibrosarcoma cells. Mol Cancer Res 4:209–220
Lin YL, Lin CY, Chi CW, Huang YT (2009) Study on antifibrotic effects of curcumin in rat hepatic stellate cells. Phytother Res 23:927–932
Lin YT, Wang LF, Hsu YC (2009) Curcuminoids suppress the growth of pharynx and nasopharyngeal carcinoma cells through induced apoptosis. J Agric Food Chem 57:3765–3770
Okunieff P, Xu J, Hu D, Liu W, Zhang L, Morrow G, Pentland A, Ryan JL, Ding I (2006) Curcumin protects against radiation-induced acute and chronic cutaneous toxicity in mice and decreases mRNA expression of inflammatory and fibrogenic cytokines. Int J Radiat Oncol Biol Phys 65:890–898
Ono K, Naiki H, Yamada M (2006) The development of preventives and therapeutics for Alzheimer’s disease that inhibit the formation of beta-amyloid fibrils (fAbeta), as well as destabilize preformed fAbeta. Curr Pharm Des 12:4357–4375
Panchatcharam M, Miriyala S, Gayathri VS, Suguna L (2006) Curcumin improves wound healing by modulating collagen and decreasing reactive oxygen species. Mol Cell Biochem 290:87–96
Perkins S, Verschoyle RD, Hill K, Parveen I, Threadgill MD, Sharma RA, Williams ML, Steward WP, Gescher AJ (2002) Chemopreventive efficacy and pharmacokinetics of curcumin in the min/+ mouse, a model of familial adenomatous polyposis. Cancer Epidemiol Biomarkers Prev 11:535–540
Phan TT, Sun L, Bay BH, Chan SY, Lee ST (2003) Dietary compounds inhibit proliferation and contraction of keloid and hypertrophic scar-derived fibroblasts in vitro: therapeutic implication for excessive scarring. J Trauma 54:1212–1224
Pratsinis H, Giannouli CC, Zervolea I, Psarras S, Stathakos D, Kletsas D (2004) Differential proliferative response of fetal and adult human skin fibroblasts to transforming growth factor-beta. Wound Repair Regen 12:374–383
Rahman I, Adcock IM (2006) Oxidative stress and redox regulation of lung inflammation in COPD. Eur Respir J 28:219–242
Rodriguez-Melendez R, Zempleni J (2009) Nitric oxide signaling depends on biotin in Jurkat human lymphoma cells. J Nutr 139:429–433
Ryu EK, Choe YS, Lee KH, Choi Y, Kim BT (2006) Curcumin and dehydrozingerone derivatives: synthesis, radiolabeling, and evaluation for beta-amyloid plaque imaging. J Med Chem 49:6111–6119
Schäffer MR, Tantry U, Thornton FJ, Barbul A (1999) Inhibition of nitric oxide synthesis in wounds: pharmacology and effect on accumulation of collagen in wounds in mice. Eur J Surg 165:262–267
Schäffer MR, Weimer W, Wider S, Stülten C, Bongartz M, Budach W, Becker HD (2002) Differential expression of inflammatory mediators in radiation-impaired wound healing. J Surg Res 107:93–100
Seifert O, Mrowietz U (2009) Keloid scarring: bench and bedside. Arch Dermatol Res 301:259–272
Sinha R, Anderson DE, McDonald SS, Greenwald P (2003) Cancer risk and diet in India. J Postgrad Med 49:222–228
Tan TW, Tsai HR, Lu HF, Lin HL, Tsou MF, Lin YT, Tsai HY, Chen YF, Chung JG (2006) Curcumin-induced cell cycle arrest and apoptosis in human acute promyelocytic leukemia HL-60 cells via MMP changes and caspase-3 activation. Anticancer Res 26:4361–4371
Thomas DW, Hopkinson I, Harding KG, Shepherd JP (1994) The pathogenesis of hypertrophic/keloid scarring. Int J Oral Maxillofac Surg 23:232–236
Wang XQ, Liu YK, Qing C, Lu SL (2009) A review of the effectiveness of antimitotic drug injections for hypertrophic scars and keloids. Ann Plast Surg 63:688–692
Yamamoto T, Eckes B, Krieg T (2000) Bleomycin increases steady-state levels of type I collagen, fibronectin and decorin mRNAs in human skin fibroblasts. Arch Dermatol Res 292(11):556–561
Zhang L, Keane MP, Zhu LX, Sharma S, Rozengurt E, Strieter RM, Dubinett SM, Huang M (2004) Interleukin-7 and transforming growth factor-beta play counter-regulatory roles in protein kinase C-delta-dependent control of fibroblast collagen synthesis in pulmonary fibrosis. J Biol Chem 279:28315–28319
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hsu, YC., Chen, MJ., Yu, YM. et al. Suppression of TGF-β1/SMAD pathway and extracellular matrix production in primary keloid fibroblasts by curcuminoids: its potential therapeutic use in the chemoprevention of keloid. Arch Dermatol Res 302, 717–724 (2010). https://doi.org/10.1007/s00403-010-1075-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00403-010-1075-y