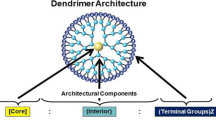
Abstract
Poly(ethyleneimine) (PEI) dendrimers up to the third generation (G3) were prepared by a divergent synthesis method from an ethylenediamine (EDA) core. The amine terminals were bonded with vinylbromide by a Michael addition reaction. Then, the bromide terminals were converted to amine groups using a Gabriel amine synthesis method. PEI dendrimers displayed pH-dependent luminescence, and their emission intensities at pH 6 increased over time. Fluorescence intensities also increased with increasing dendrimer generation from G1 to G3. Air-bubbling in aqueous solutions of dendrimers made to incorporate detectable amount of oxygen in dendrimers. EDA also behaved similarly in luminescence and oxygen incorporation.

Synthesis and Characterization of Poly(ethyleneimine) Dendrimers
Omprakash Yemul and Toyoko Imae*





Similar content being viewed by others
References
Fréchet JMJ, Hawker CJ (1996) In: Allen G (ed) Comprehensive polymer science, 2nd suppl. Elsevier Science, Pergamon, Oxford
Fischer M, Vögtle F (1999) Angew Chem Int Ed 38:884
Imae T (2003) In Esumi K, Ueno M (ed) Structure-performance relationships in surfactants: 2nd Edn, Revised and Expanded. Surfactant Science Series volume 112, Marcel Dekker, 525
Tomalia DA (2005) Chimica OGGI-Chem Today 23:41
Zeng F, Zimmerman SC (1997) Chem Rev 97:1681
Imae T, Funayama K, Aoi K, Tsutsumiuchi K, Okada M, Furusaka M (1999) Langmuir 15:4076
Percec V, Holerca MN (2000) Biomolecules 1:6
Adronov A, Frechet JM (2000) Chem Commun 1701
Lee I, Athey BD, Wetzel AW, Meixner W, Baker JR Jr (2002) Macromolecules 35:4510
Hedden RC, Bauer BJ (2003) Macromolecules 36:1829
Funayama K, Imae T, Aoi K, Tsutsumiuchi K, Okada M, Furusaka, M, Nagao M (2003) J Phys Chem B 107:1532
Imae T (2004) In: Nalwa HS (ed) Encyclopedia of nanoscience and nanotechnology. American Scientific, 3, p 685
Ujihara M, Mitamura K, Torikai N, Imae T (2006) Langmuir 22:3656
Godbey WT, Wu KK, Mikos AG (1999) J Control Release 60:149
Li K, Geng X, Simonsen J, Karchesy J (2004) Int J Adhesion Adhesive 24:327
Tomalia DA, Baker H, Dewald J, Hall M, Kallos G, Martin S, Roeck J, Ryder J, Smith P (1986) Macromolecules 19:2466
Aoi K, Motoda A, Ohno M, Tsutsumiuchi K, Okada M, Imae T (1997) Polymer J 31:1071
Aoi K, Motoda A, Okada M, Imae T (1997) Macromol Rapid Commun 18:945
Hawker CJ, Fréchet JMJ (1990) J Am Chem Soc 112:7638
Wooley WKL, Hawker CJ, Fréchet JMJ (1991) J Am Chem Soc 113:4252
Wörner C, Mülhaupt R (1993) Angew Chem Int Ed Engl 32:1306
de Brabander-van den Berg EMM, Meijer EW (1993) Angew Chem Int Ed Engle 32:1308
Ikeda Y, Imae T, Hao J, Iida M, Kitan T (2000) Langmuir 16:7618
Pistolis G, Malliaris A, Paleos CM, Tsiourvas D (1997) Langmuir 13:5870
Jockusch S, Ramirez J, Sanghvi K, Nociti R, Turro NJ, Tamalia DA (1999) Macromolecules 32:4419
Wade DA, Torres PA, Tucker SA (1999) Anal Chim Acta 397:17
Sideratou Z, Tsiouras D, Paleos CM (2000) Langmuir 16:1766
Richter-Egger DL, Tesfai A, Tucker SA (2001) Anal Chem 73:5743
Varnavski O, Ispasoiu RG, Balogh L, Tomalia D, Goodson T III (2001) J Chem Phys 114:1962
Larson CL, Tucker SA (2001) Appl Spectrosc 55:679
Lee WI, Bae Y, Bard AJ (2004) J Am Chem Soc 126:8358
Wang D, Imae T (2004) J Am Chem Soc 126:13204
Wang D, Imae T (2007) J Colloid Interface Sci 306:222
Wu D, Liu Y, He C, Goh SH (2005) Macromolecules 38:9906
Kirchner K, Merget N, Schmidt C (1974) Chem Ing Tech 46:661
Patai S (Ed) (1982) The chemistry of amino, nitroso and nitro compounds and their derivatives, part 2. Patai series, Wiley, New York
Blaschko H (1953) Enzymic Brit Med Bull 9:146
Acknowledgments
The authors are thankful to Dr. Y. Maeda in the Nagoya University for providing NMR spectra of samples. OY is grateful to the 21st Century COE Program (No. 14COEB01-00) for financial support of postdoctoral fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yemul, O., Imae, T. Synthesis and characterization of poly(ethyleneimine) dendrimers. Colloid Polym Sci 286, 747–752 (2008). https://doi.org/10.1007/s00396-007-1830-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-007-1830-6