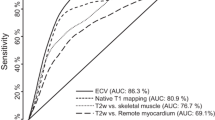
Abstract
Introduction
This study evaluated the ability of T1 and T2 mapping cardiovascular magnetic resonance (CMR) to detect myocardial injury in apparently normal myocardium of patients with myocarditis.
Materials and methods
We included 20 patients with “infarct-like” acute myocarditis who had typical focal myocardial lesions on late gadolinium enhancement (LGE) images as well as 20 healthy controls. The CMR protocol consisted of a standard myocarditis protocol which was combined with T1 (modified Look-Locker inversion recovery (MOLLI) with a 3(3)5 scheme and T2 mapping (hybrid gradient- and spin-echo multi-echo sequence, GraSE). First, LGE images were used to depict focal myocardial injury and apparently normal, remote myocardium. Second, native T1, T2 and ECV values were obtained in focal lesions but also in apparently normal myocardium. Third, native T1, T2 and ECV values ≥2 standard deviations above reference values obtained in healthy volunteers were used to quantify myocardial injury in patients with myocarditis.
Results
Apparently normal myocardium had significantly higher median native T1 [1095 (1055–1148) ms] and ECV [34 (32–35) %] values compared to reference values from healthy volunteers, which were 1051 (1021–1064) ms (p < 0.01) and 26 (24–27) % (p < 0.0001). Furthermore, a nonsignificant increase in median myocardial T2 was detected in apparently normal myocardium of patients with myocarditis compared to healthy volunteers [59 (55–65) vs. 56 (54–60) ms; p = 0.18]. Consequently, the amount of myocardial injury was significantly larger on native T1 [48 (32–56) %; p < 0.01] and ECV maps [58 (50–66) %; p < 0.01] compared to LGE [14 (9–20) %].
Conclusions
Native T1 and ECV maps reveal hidden myocardial injury in normal appearing myocardium of patients with myocarditis. The amount of myocardial injury in myocarditis was underestimated by conventional LGE imaging.
Similar content being viewed by others
Introduction
Myocarditis is frequently clinically defined, but endomyocardial biopsy is currently perceived as the “gold standard”, in particular, in subgroups who might benefit from specific therapy [1–6]. In recent years, cardiovascular magnetic resonance (CMR) imaging emerged as an important diagnostic tool in the diagnostic work-up of patients with suspected myocarditis, especially in young patients presenting with “infarct-like” symptoms and raised cardiac enzymes [7–10]. In particular, the proof of focal myocardial injury on late gadolinium enhancement (LGE) and T2-weighted (T2w) CMR images is currently a crucial criterion for diagnosing myocarditis by CMR [11]. Focal myocardial injury is typically defined on LGE and T2w images by an increased signal intensity compared to normal appearing myocardium as reference tissue [12–14]. However, this semi-quantitative standard method is prone to errors due to the arbitrary definition of normal appearing myocardium as reference tissue, which could include unapparent myocardial injury, and thereby hampering an accurate assessment of myocardial injury [13]. This appears to be of particular importance in patients with myocarditis since intensity and distribution of inflammatory infiltrates are highly variable ranging from solitary small foci to a diffuse involvement of the entire myocardium [15, 16]. T1 and T2 mapping CMR are emerging quantitative techniques for the assessment of myocardial injury without the need for arbitrarily defined reference tissue [17–20]. Myocardial T2 maps reflect free tissue water content and can be used to detect myocardial edema [21–23]. Native myocardial T1 maps reflect several alterations in myocardial tissue composition, such as edema [23–25], necrosis and fibrosis [25–27]. A combination of native and post contrast T1 maps enable quantification of the extracellular volume (ECV) fraction, which provides further information about the extent of myocardial injury in several cardiac diseases [18, 19, 28]. Recent studies indicate that these mapping techniques may improve the diagnostic accuracy of CMR in patients with suspected myocarditis [22, 29–31]. In particular, Ferreira et al. [32] recently demonstrated the incremental value of native T1 mapping to assess focal myocardial injury in myocarditis. This study evaluated the ability of native myocardial T1, T2 and ECV maps to detect myocardial injury in apparently normal myocardium of patients with myocarditis.
Materials and methods
Patients and healthy volunteers
The local ethics committee approved the study and all subjects gave their written informed consent. Our inclusion criteria were clinically defined myocarditis with history of recent airway and/or gastrointestinal infection, dynamically elevated Troponin T and acute chest pain in combination with positive “Lake Louise Criteria”, mandatorily including typical unambiguously nonischemic LGE patterns as the CMR reference method for focal myocardial injury [33]. 30 patients with clinically suspected “infarct-like” myocarditis were screened for this study. Ten patients were excluded due to absent or ambiguous LGE as well as for having lesions on LGE images (full coverage) without available corresponding native T1, myocardial T2 and ECV maps (three slices only). Finally, the study population included 20 patients with myocarditis and a control group of 20 healthy volunteers. Clinical data of some of the patients and reference values from healthy volunteers were previously published in a study with a different focus [31]. This study did not include patients who presented with symptoms of heart failure or arrhythmia since these patients display more diffuse patterns of myocardial injury [34], which were not the focus of this study. CMR was performed at median of 4 days (IQR 3–10 days) after onset of symptoms. 18 (90 %) patients presented with electrocardiogram abnormalities. Four patients (20 %) had ST segment elevation, five patients (25 %) had ST segment depression, 14 patients (70 %) had T-wave inversion and one patient (5 %) had a left bundle branch block. Coronary artery disease was excluded in 12 patients (60 %) by coronary angiography as clinically indicated; the other patients did not undergo coronary angiography due to an individually extremely low likelihood of coronary artery disease. Age and gender of patients and healthy volunteers did not differ significantly. Healthy volunteers had no history of any cardiovascular disease, risk factors or cardiovascular medication, with normal cardiac biomarkers and electrocardiograms. The major clinical characteristics of patients and healthy volunteers are presented in Table 1.
CMR protocol
CMR was performed on a 1.5 T scanner (Achieva, Philips Medical Systems, Best, The Netherlands) using ECG-triggered and either breath held or respiratory navigator-gated sequences. The body coil was used as transmitter and a phased array surface coil was used as receiver for signal intensity based sequences. Surface coil related signal inhomogeneity was corrected by an inherent signal intensity correction algorithm (constant level appearance, CLEAR). A retrospectively gated cine-CMR short axis stack was acquired to obtain left ventricular volumes and function using a steady-state free precession (SSFP) sequence with the following typical imaging parameters: Acquired voxel size (AVS) 2.16 × 2.08 × 8 mm3, reconstructed voxel size (RVS) 1.46 × 1.47 × 8 mm3, echo time (TE) = 1.67 ms, time to repetition (TR) = 3.34 ms, flip angle = 60°, parallel acquisition technique = SENSE. Edema-sensitive T2-weighted (T2w) CMR was performed on end-diastolic left ventricular short axes using a fat suppressed (short inversion time inversion recovery, STIR), black blood triple inversion recovery turbo-spin-echo sequence with the following typical imaging parameters: AVS 1.65 × 1.75 × 10 mm3, 1.65 × 1.75 × 10 mm3, RVS 1.45 × 1.44 × 10 mm3, TE = 90 ms, 25 echoes, TR = 1600 ms. A bolus of 0.075 mmol/kg gadobenate dimeglumine was injected at a rate of 2.5 ml/s. Phase-sensitive Inversion Recovery (PSIR) LGE imaging was performed on end-diastolic short axes with the following typical imaging parameters: AVS 1.99 × 2.13 × 6 mm3, RVS 1.35 × 1.36 × 6 mm3, TE = 2.53 ms, TR = 5.21 ms, flip angle = 15°.
In addition to this routine protocol, T2 and T1 mapping were performed on three representative short axes slices (basis, center, apex) with identical slice positions compared to respective LGE and T2w slices: T2-mapping was performed before administration of contrast media using a BB prepared, navigator-gated, free-breathing hybrid gradient (echo planar imaging, EPI) and spin-echo multi-echo sequence (GraSE) with the following typical imaging parameters: AVS 2.01 × 2.01 × 10 mm3, RVS 1.05 × 1.05 × 10 mm3, nine readouts with effective TE between 12.5 and 62.4 ms, TR = 1600 ms. T1 mapping was performed using the 3(3)5 modified Look-Locker inversion recovery (MOLLI) sequence before and 15 min after contrast media administration. Typical imaging parameters were as follows: AVS 1.70 × 2.15 × 10 mm3, RVS 1.19 × 1.19 × 10 mm3, eight single shot balanced SSFP readout using a 3(3)5 scheme, TE = 1.59 ms, TR = 3.17 ms, flip angle = 35°, SENSE factor = 2, linear phase encoding, ten startup cycles to approach steady-state prior to imaging, effective inversion times between 188 and 3382 ms [31].
Generation of T1, T2 and ECV maps
T2 maps were generated from the nine acquired echoes per short axis slice using a dedicated plug-in written for the OsiriX™ software as described before [31]. Endo- and epicardial contours were manually drawn and propagated through the image stack. Contours were manually corrected and aligned with the contours in each respective component image. Similarly, T1 maps were generated from the eight acquired images per slice using another dedicated plug-in written for the OsiriX™ software as described before [31]. A rigid body transformation matrix was computed including translation in both in-plane directions and employed in the T1 fitting procedure to compensate for breathing-related shifts. ECV maps were generated by the software from native and post contrast T1 maps including the haematocrit, which was measured from a venous blood sample taken on the same day of CMR performance as described before [31]. The recommended phantom studies for validation of our T1 and T2 mapping method were recently published [29, 31]. LGE and T2w short axis slices, native T1, T2 and ECV maps were uploaded in heart analysis tool (HeAT), dedicated in-house CMR data analysis software for quantifying myocardial injury [35].
Myocardial injury on LGE and T2w images
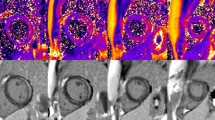
Two experienced observers performed the CMR data analyses. Myocardial injury was quantified using a standard threshold technique relative to normal appearing myocardium using the HeAT software on LGE and T2w images (Fig. 1): a region of interest was drawn in normal appearing, LGE negative myocardium on LGE images and then propagated to corresponding T2w images. Injured myocardium was then defined by a mean signal intensity ≥2 standard deviations above normal appearing, LGE negative myocardium as recently established in similar populations [12, 14, 32]. The amount of myocardial injury was calculated as the percentage of pixels with injured myocardium.
Example for quantification of focal myocardial injury in a patient with myocarditis using normal appearing, remote myocardium as reference on LGE (a) and T2w CMR (b) images and using myocardium of healthy volunteers as reference tissue on native T1 (c), T2 (d) and ECV maps (e). Injured myocardium in LGE (a) and T2w CMR (b) images is visualized by light green [≥2 SD above mean signal intensity (SI) of reference region of interest] to light blue (≥5 SD above mean SI of reference region of interest). Injured myocardium on native T1 (c), T2 (d) and ECV maps (e) (red) was defined by native T1, T2 or ECV values ≥2 SD above reference myocardium of healthy controls
Myocardial injury on native T1, T2 and ECV maps
Two different approaches were applied for defining focal lesions on native T1, T2 and ECV maps: first, focal LGE lesions were defined by a threshold method, using a signal intensity ≥2 standard deviations above the mean value of normal appearing, remote myocardium on LGE images to depict injured myocardium. The region of interest in remote myocardium was then transferred from LGE images to corresponding native T1, T2 and ECV maps, where lesions were similarly defined by native T1, T2 and ECV values ≥2 standard deviations above the mean value of remote myocardium. Second, a more simple approach was applied by manually drawing a region of interest around LGE positive myocardial lesions. This lesion contour was transferred to corresponding native T1, T2 and ECV maps. Both methods were used to measure native T1, T2 and ECV in focal myocardial lesions and in apparently healthy myocardium. These values were then compared with reference values of healthy controls. Finally, the amount of myocardial injury was defined by native T1, T2 and ECV values ≥2 SD above reference values from healthy controls. The amount of myocardial injury was calculated as the percentage of pixels with injured myocardium similar to the signal intensity based approach on conventional LGE and T2w images (Fig. 1).
Statistical analysis
Statistical analyses were performed using GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, CA, USA) and MedCalc for Windows, version 13.3.3.0 (MedCalc Software, Ostend, Belgium). Continuous data are presented as median with first and third quartiles. Categorical data are presented as numbers and percentage. Continuous data were compared using the nonparametric Mann–Whitney test. Categorical data were compared using Fisher’s exact test. Statistical significance was set to p < 0.05.
Results
Regional native T1, ECV and T2 values
Figure 2 demonstrates native T1, T2 and ECV values in focal lesions, apparently normal myocardium and in healthy volunteers as defined by the threshold method 1. Lesions had significantly higher median native T1 [1448 (1406–1606) vs. 1095 (1055–1148) ms], ECV [56 (52–64) vs. 34 (32–35) %] and T2 values [93 (85–114) vs. 59 (55–65) ms] compared with apparently normal myocardium (all values <0.0001). Apparently normal myocardium showed significantly increased values of median native T1 (p < 0.01) and ECV (p < 0.0001) compared to myocardium of healthy volunteers, who had a median native T1 of 1051 (1021–1064) ms and a median ECV of 26 (24–27) %, respectively. Furthermore, a nonsignificant elevation of myocardial T2 was found in apparently normal myocardium compared with the reference value in healthy volunteers [56 (54–60) ms; p = 0.18]. Similar to the threshold approach, manually defined LGE positive lesions showed significantly higher values for median native T1 [1228 (1156–1292) ms; p < 0.01], ECV [42 (38–47) %; p < 0.001] and myocardial T2 [68 (63–88) ms; p < 0.01] in comparison to normal appearing myocardium as well as in comparison to native T1, ECV and myocardial T2 (all values <0.0001) in healthy controls.
Quantification of myocardial injury
Figure 3 demonstrates the amount of myocardial injury quantified on LGE images, T2w images, native T1 maps, ECV maps and T2 maps. The threshold values (≥2 SD relative to normal values in healthy controls) were 1120 ms on native T1 maps, ≥31 % on ECV maps and ≥65 ms on T2 maps, respectively. Median lesion size was 14 (9–20 %) on LGE images as the reference technique and 9 (4–23 %) on T2w images (p = 0.78). The largest myocardial injury of the applied mapping methods was found on ECV maps [58 (50–66) %] compared with native T1 maps [48 (32–56) %; p < 0.05], T2 maps [18 (9–38) %; p < 0.01], LGE images and T2w images (p < 0.01). Furthermore, the amount of myocardial injury was also significantly larger on native T1 maps [48 (32–56) %] compared to LGE images and T2w images (p < 0.01). A nonsignificant difference in myocardial injury was found between T2 maps [18 (9–38) %] compared with LGE (p = 0.06) and T2w images (p = 0.05).
The green box plots show a median amount of myocardial injury of 14 (9–20) % on LGE images and 9 (4–23) % on T2w images using normal appearing, remote myocardium of patients with myocarditis as reference tissue. The red box plots show a median amount of myocardial injury of 18 (9–38) % on T2 maps, 48 (32–56) % on native T1 maps and 58 (50–66) % on ECV maps using myocardium of healthy volunteers as reference tissue
Discussion
This study provides the first head-to-head comparison of conventional LGE images with native T1, T2 and ECV mapping techniques for characterizing focal myocardial injury in patients with myocarditis. The major findings were:
-
Focal lesions were characterized by a strong increase in native T1, ECV and T2 (Fig. 2).
-
Normal appearing myocardium also had increased native T1, ECV, and to a lesser degree, T2 values (Fig. 2).
-
Consequently, the amount of myocardial injury was significantly larger on native T1 and ECV maps compared with conventional LGE images (Fig. 3).
LGE is currently the reference method to assess myocardial necrosis and/or replacement fibrosis by CMR and carries important diagnostic but also prognostic information in patients with myocarditis. We used a threshold method relative to normal appearing myocardium to depict myocardial injury on LGE images. This standard signal intensity based approach was adapted from reference methods to quantify myocardial scar in ischemic heart disease and established in myocarditis by other groups [12, 14, 32, 36–38]. Median myocardial injury was 14 (9–20) % on LGE images in agreement with recent data by Ferreira et al., who reported LGE lesion sizes of 16 (8–24) % with a similar approach and in a similar cohort of patients with myocarditis [32]. Major advantage of novel quantitative T1 and T2 mapping techniques compared to signal intensity based techniques such as LGE is that “true” normal values can be obtained in healthy individuals as reference tissue instead of normal appearing myocardium in diseased patients. We found significantly increased native T1 and ECV values in normal appearing myocardium of patients with myocarditis as compared to normal myocardium of healthy volunteers (Fig. 2). Consequently, we found significantly larger amounts of myocardial injury on native T1 and ECV maps compared to LGE images (Fig. 3). This finding indicates the presence of unapparent myocardial injury in normal appearing, LGE negative regions. Although myocarditis may be limited to solitary small myocardial foci [16], our results underscore that myocarditis is principally a diffuse disease despite focal appearance on LGE images. Therefore, the use of normal appearing myocardium as reference tissue on LGE images can result in a systematic underestimation of the true amount of myocardial injury. Nevertheless, the exact histological difference between LGE and focal lesions on native T1, T2 or ECV maps is not fully understood and could be of different prognostic value. However, the larger lesion size on native T1 and ECV maps is most likely related to higher sensitivity of mapping methods, allowing the detection of mildly injured myocardium. Our findings agree well with recent data by Ferreira et al. [32], who demonstrated myocardial injury on native T1 maps reaching beyond LGE lesions in patients with myocarditis. However, our threshold value for native T1 differed significantly. This discrepancy can be explained by two reasons: First, we defined injured myocardium by increased native T1, T2 and ECV values ≥2 SD above normal values from healthy volunteers. In contrast, the threshold of Ferreira et al. [32] was established in patients with takotsubo cardiomyopathy and optimized to differentiate involved from uninvolved myocardial segments. Second, different normal values in the control groups of both studies highlight the systematic methodological differences in T1 mapping techniques. We have recently validated our approach of T1 quantification in a phantom study and obtained institutional normal values in healthy controls as recommended [31]. Furthermore, other groups found similar native T1 values on the same 1.5 Tesla scanner [39]. This issue underscores that different mapping sequences (ShMOLLI vs. MOLLI), imaging parameters, such as slice thickness but also post processing issues such as the fitting model for T1 estimation may affect native T1 normal values [40]. Therefore, site specific validations in phantoms and healthy controls are necessary and recommended in current guidelines [19]. Notwithstanding, both studies are essentially in line despite different normal values and underscore the incremental diagnostic value of native myocardial T1 in myocarditis.
Interestingly, T2 values in normal appearing myocardium were altered to a lesser degree compared to native T1 and ECV values (Fig. 2). This finding agrees well with Thavendiranathan et al., who did not find significant differences in myocardial T2 between normal appearing myocardium of patients with myocarditis and myocardium of healthy controls [22]. This observation could be related to several reasons: First, native myocardial T1 and ECV could be more sensitive for myocardial injury in myocarditis or less prone to artifacts compared with myocardial T2-weighted, but also T2 mapping CMR. Second, there could be a principally different pattern of myocardial injury in LGE negative, apparently healthy areas such as early myocardial fibrosis without active inflammation. Third, myocardial edema could be unstable in myocarditis similar to recent observations in experimental myocardial infarction, which could result in intermittent normalization of myocardial T2 values [41]. Fourth, any threshold technique introduces arbitrariness into data analysis. Our threshold of T2 values ≥65 ms matches well with LGE, but could be not sensitive enough. Using different approaches to define the T2 threshold such as Thavendiranathan et al. who used a T2 value ≥59 ms would have led to different results [22]. Importantly, threshold values for diffuse, global myocardial injury in patients with “cardiomyopathy-like” myocarditis appear to be different from thresholds for focal myocardial injury and may not be transferred between different subgroups of myocarditis [29]. In conclusion, further studies with histological correlation by targeted biopsies are necessary to fully understand the regional CMR characteristics of myocardial injury in myocarditis.
Limitations
Coronary artery disease was not excluded by clinically indicated coronary angiography in 40 % of our patients, who had a very low likelihood of coronary artery disease due to young age and/or the lack of any cardiovascular risk factors. However, we cannot completely exclude the presence of concomitant coronary artery disease as a potential confounder in these patients. Furthermore, the diagnosis of myocarditis was clinically defined and not by endomyocardial biopsy. Nevertheless, recent findings support the validity of this approach in the subgroup of patients with “infarct-like” myocarditis, whereas endomyocardial biopsy would have been necessary in other subgroups of myocarditis [34]. In contrast to recent studies, we did not use a minimal contiguous area of adjacent pixels to define myocardial injury [32]. We therefore cannot exclude that noise could have led to overestimation of lesions size. However, lesion sizes in our study were similar to data by Ferreira et al. [32] who included only areas with ten adjacent pixels for defining myocardial injury. Using the surface coil as receiver for the T2w STIR sequence constitutes a potential source of error for the assessment of focal myocardial injury [11]. Therefore, we used an inherent signal intensity correction algorithm (constant level appearance, CLEAR) to overcome this potential limitation. Furthermore, we cannot exclude confounding effects of epicardial fat on T1 values measured by our SSFP based MOLLI variant as recently indicated by Kellman et al. [42].
Conclusion
Native T1 and ECV maps reveal hidden myocardial injury in normal appearing myocardium of patients with myocarditis. The amount of myocardial injury in myocarditis was underestimated by conventional LGE imaging.
Abbreviations
- AVS:
-
Acquired voxel size
- CMR:
-
Cardiovascular magnetic resonance
- ECV:
-
Extracellular volume
- HeAT:
-
Heart analysis tool
- IQR:
-
Interquartile range
- LGE:
-
Late gadolinium enhancement
- MOLLI:
-
Modified Look-Locker inversion recovery
- RVS:
-
Reconstructed voxel size
- SFP:
-
Steady-state free precession
- T2w:
-
T2-weighted
References
Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, Felix SB, et al. (2013) Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 34(33):2636–2648, 48a–48d
Elezkurtaj S, Lassner D, Schultheiss HP, Escher F (2014) Vascular involvement in cardiac giant cell myocarditis: a new pathophysiological aspect. Clin Res Cardiol 103(2):161–163
Greulich S, Kindermann I, Schumm J, Perne A, Birkmeier S, Grun S et al (2016) Predictors of outcome in patients with parvovirus B19 positive endomyocardial biopsy. Clin Res Cardiol 105(1):37–52
Schultheiss HP, Piper C, Sowade O, Waagstein F, Kapp JF, Wegscheider K, et al. (2016) Betaferon in chronic viral cardiomyopathy (BICC) trial: effects of interferon-beta treatment in patients with chronic viral cardiomyopathy. Clin Res Cardiol. doi:10.1007/s00392-016-0986-9
Weitsman T, Weisz G, Keren A, Hasin T (2016) Prompt benefit of early immunosuppressive therapy in acute lymphocytic myocarditis with persistent heart failure. Clin Res Cardiol. doi:10.1007/s00392-016-0985-x
Zuern CS, Walker B, Sauter M, Schaub M, Chatterjee M, Mueller K et al (2015) Endomyocardial expression of SDF-1 predicts mortality in patients with suspected myocarditis. Clin Res Cardiol 104(12):1033–1043
Illmann A, Riemer T, Erbel R, Giannitsis E, Hamm C, Haude M et al (2014) Disease distribution and outcome in troponin-positive patients with or without revascularization in a chest pain unit: results of the German CPU-Registry. Clin Res Cardiol 103(1):29–40
Poryo M, Khreish F, Schafers HJ, Abdul-Khaliq H (2016) A case of myocardial bridging as a rare cause of chest pain in children. Clin Res Cardiol 105(3):279–281
Ukena C, Kindermann M, Mahfoud F, Geisel J, Lepper PM, Kandolf R et al (2014) Diagnostic and prognostic validity of different biomarkers in patients with suspected myocarditis. Clin Res Cardiol 103(9):743–751
Florian A, Schaufele T, Ludwig A, Rosch S, Wenzelburger I, Yildiz H et al (2015) Diagnostic value of CMR in young patients with clinically suspected acute myocarditis is determined by cardiac enzymes. Clin Res Cardiol 104(2):154–163
Friedrich MG, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P, Cooper LT et al (2009) Cardiovascular magnetic resonance in myocarditis: a JACC white paper. J Am Coll Cardiol 53(17):1475–1487
Baccouche H, Mahrholdt H, Meinhardt G, Merher R, Voehringer M, Hill S et al (2009) Diagnostic synergy of non-invasive cardiovascular magnetic resonance and invasive endomyocardial biopsy in troponin-positive patients without coronary artery disease. Eur Heart J 30(23):2869–2879
Vermes E, Childs H, Carbone I, Barckow P, Friedrich MG (2013) Auto-threshold quantification of late gadolinium enhancement in patients with acute heart disease. J Magn Reson Imaging JMRI 37(2):382–390
Zagrosek A, Abdel-Aty H, Boye P, Wassmuth R, Messroghli D, Utz W et al (2009) Cardiac magnetic resonance monitors reversible and irreversible myocardial injury in myocarditis. JACC Cardiovasc Imaging 2(2):131–138
Friedrich MG, Strohm O, Schulz-Menger J, Marciniak H, Luft FC, Dietz R (1998) Contrast media-enhanced magnetic resonance imaging visualizes myocardial changes in the course of viral myocarditis. Circulation 97(18):1802–1809
Basso C, Calabrese F, Angelini A, Carturan E, Thiene G (2013) Classification and histological, immunohistochemical, and molecular diagnosis of inflammatory myocardial disease. Heart Fail Rev 18(6):673–681
aus dem Siepen F, Buss SJ, Andre F, Seitz S, Giannitsis E, Steen H et al (2015) Extracellular remodeling in patients with wild-type amyloidosis consuming epigallocatechin-3-gallate: preliminary results of T1 mapping by cardiac magnetic resonance imaging in a small single center study. Clin Res Cardiol 104(8):640–647
Ferreira VM, Piechnik SK, Robson MD, Neubauer S, Karamitsos TD (2014) Myocardial tissue characterization by magnetic resonance imaging: novel applications of T1 and T2 mapping. J Thorac Imaging 29(3):147–154
Moon JC, Messroghli DR, Kellman P, Piechnik SK, Robson MD, Ugander M et al (2013) Myocardial T1 mapping and extracellular volume quantification: a Society for Cardiovascular Magnetic Resonance (SCMR) and CMR Working Group of the European Society of Cardiology consensus statement. J Cardiovasc Magn Reson Off J Soc Cardiovasc Magn Reson 15:92
Schumm J, Greulich S, Sechtem U, Mahrholdt H (2014) T1 mapping as new diagnostic technique in a case of acute onset of biopsy-proven viral myocarditis. Clin Res Cardiol 103(5):405–408
Giri S, Chung YC, Merchant A, Mihai G, Rajagopalan S, Raman SV et al (2009) T2 quantification for improved detection of myocardial edema. J Cardiovas Magn Reson Off J Soc Cardiovas Magn Reson 11:56
Thavendiranathan P, Walls M, Giri S, Verhaert D, Rajagopalan S, Moore S et al (2012) Improved detection of myocardial involvement in acute inflammatory cardiomyopathies using T2 mapping. Circ Cardiovas Imaging 5(1):102–110
Ugander M, Bagi PS, Oki AJ, Chen B, Hsu LY, Aletras AH et al (2012) Myocardial edema as detected by pre-contrast T1 and T2 CMR delineates area at risk associated with acute myocardial infarction. JACC Cardiovas Imaging 5(6):596–603
Ferreira VM, Piechnik SK, Dall’Armellina E, Karamitsos TD, Francis JM, Choudhury RP et al (2012) Non-contrast T1-mapping detects acute myocardial edema with high diagnostic accuracy: a comparison to T2-weighted cardiovascular magnetic resonance. J Cardiovas Magn Reson Off J Soc Cardiovas Magn Reson 14:42
Messroghli DR, Niendorf T, Schulz-Menger J, Dietz R, Friedrich MG (2003) T1 mapping in patients with acute myocardial infarction. J Cardiovas Magn Reson Off J Soc Cardiovas Mag Reson 5(2):353–359
Bull S, White SK, Piechnik SK, Flett AS, Ferreira VM, Loudon M et al (2013) Human non-contrast T1 values and correlation with histology in diffuse fibrosis. Heart 99(13):932–937
Iles L, Pfluger H, Phrommintikul A, Cherayath J, Aksit P, Gupta SN et al (2008) Evaluation of diffuse myocardial fibrosis in heart failure with cardiac magnetic resonance contrast-enhanced T1 mapping. J Am Coll Cardiol 52(19):1574–1580
Arheden H, Saeed M, Higgins CB, Gao DW, Bremerich J, Wyttenbach R et al (1999) Measurement of the distribution volume of gadopentetate dimeglumine at echo-planar MR imaging to quantify myocardial infarction: comparison with 99mTc-DTPA autoradiography in rats. Radiology 211(3):698–708
Bohnen S, Radunski UK, Lund GK, Kandolf R, Stehning C, Schnackenburg B, et al. (2015) Performance of t1 and t2 mapping cardiovascular magnetic resonance to detect active myocarditis in patients with recent-onset heart failure. Circ Cardiovas Imaging 8(6). doi:10.1161/CIRCIMAGING.114.003073
Ferreira VM, Piechnik SK, Dall’Armellina E, Karamitsos TD, Francis JM, Ntusi N et al (2013) T(1) mapping for the diagnosis of acute myocarditis using CMR: comparison to T2-weighted and late gadolinium enhanced imaging. JACC Cardiovas Imaging 6(10):1048–1058
Radunski UK, Lund GK, Stehning C, Schnackenburg B, Bohnen S, Adam G et al (2014) CMR in patients with severe myocarditis: diagnostic value of quantitative tissue markers including extracellular volume imaging. JACC Cardiovas Imaging 7(7):667–675
Ferreira VM, Piechnik SK, Dall’Armellina E, Karamitsos TD, Francis JM, Ntusi N et al (2014) Native T1-mapping detects the location, extent and patterns of acute myocarditis without the need for gadolinium contrast agents. J Cardiovas Magn Reson Off J Soc Cardiovas Magn Reson 16:36
Abdel-Aty H, Boye P, Zagrosek A, Wassmuth R, Kumar A, Messroghli D et al (2005) Diagnostic performance of cardiovascular magnetic resonance in patients with suspected acute myocarditis: comparison of different approaches. J Am Coll Cardiol 45(11):1815–1822
Francone M, Chimenti C, Galea N, Scopelliti F, Verardo R, Galea R et al (2014) CMR sensitivity varies with clinical presentation and extent of cell necrosis in biopsy-proven acute myocarditis. JACC Cardiovas Imaging 7(3):254–263
Saring D, Ehrhardt J, Stork A, Bansmann MP, Lund GK, Handels H (2006) Computer-assisted analysis of 4D cardiac MR image sequences after myocardial infarction. Methods Inf Med 45(4):377–383
Kim RJ, Fieno DS, Parrish TB, Harris K, Chen EL, Simonetti O et al (1999) Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation 100(19):1992–2002
Lund GK, Stork A, Saeed M, Bansmann MP, Gerken JH, Muller V et al (2004) Acute myocardial infarction: evaluation with first-pass enhancement and delayed enhancement MR imaging compared with 201Tl SPECT imaging. Radiology 232(1):49–57
Mahrholdt H, Wagner A, Holly TA, Elliott MD, Bonow RO, Kim RJ et al (2002) Reproducibility of chronic infarct size measurement by contrast-enhanced magnetic resonance imaging. Circulation 106(18):2322–2327
Roujol S, Weingartner S, Foppa M, Chow K, Kawaji K, Ngo LH et al (2014) Accuracy, precision, and reproducibility of four T1 mapping sequences: a head-to-head comparison of MOLLI, ShMOLLI, SASHA, and SAPPHIRE. Radiology 272(3):683–689
Kellman P, Hansen MS (2014) T1-mapping in the heart: accuracy and precision. J Cardiovas Magn Reson Off J Soc Cardiovas Magn Reson 16:2
Fernandez-Jimenez R, Sanchez-Gonzalez J, Aguero J, Garcia-Prieto J, Lopez-Martin GJ, Garcia-Ruiz JM et al (2015) Myocardial edema after ischemia/reperfusion is not stable and follows a bimodal pattern: imaging and histological tissue characterization. J Am Coll Cardiol 65(4):315–323
Kellman P, Bandettini WP, Mancini C, Hammer-Hansen S, Hansen MS, Arai AE (2015) Characterization of myocardial T1-mapping bias caused by intramyocardial fat in inversion recovery and saturation recovery techniques. J Cardiovas Magn Reson Off J Soc Cardiovas Magn Reson 17:33
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Christian Stehning is an employee of Philips Research Germany and Dr. Schnackenburg is an employee of Philips Healthcare Germany. The other authors do not have relationships relevant to the contents of this paper to disclose.
Rights and permissions
About this article
Cite this article
Radunski, U.K., Lund, G.K., Säring, D. et al. T1 and T2 mapping cardiovascular magnetic resonance imaging techniques reveal unapparent myocardial injury in patients with myocarditis. Clin Res Cardiol 106, 10–17 (2017). https://doi.org/10.1007/s00392-016-1018-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-016-1018-5