Abstract
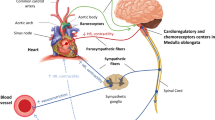
It has been extensively demonstrated that an elevated heart rate is a modifiable, independent risk factor for cardiovascular events. A high heart rate increases myocardial oxygen consumption and reduces diastolic perfusion time. It can also increase ventricular diastolic pressures and induce ventricular arrhythmias. Critical care patients are prone to develop a stress induced cardiac impairment and consequently an increase in sympathetic tone. This in turn increases heart rate. In this setting, however, heart rate lowering might be difficult because the effects of inotropic drugs could be hindered by heart rate reducing drugs like beta-blockers. Ivabradine is a new selective antagonist of funny channels. It lowers heart rate, reducing the diastolic depolarization slope. Moreover, ivabradine is not active on sympathetic pathways, thus avoiding any interference with inotropic amines. We reviewed the literature available regarding heart rate control in critical care patients, focusing our interest on the use of ivabradine to assess the potential benefits of the drug in this particular setting.


Similar content being viewed by others
References
Gillman MW, Kannel WB, Belanger A, D’Agostino RB (1993) Influence of heart rate on mortality among persons with hypertension: the Framingham Study. Am Heart J 125:1148–1154
Chang M, Havlik RJ, Corti MC, Chaves PH, Fried LP, Guralnik JM (2003) Relation of heart rate at rest and mortality in the Women’s Health and Aging Study. Am J Cardiol 92:1294–1299
Kannel WB, Kannel C, Paffenbarger RS Jr, Cupples LA (1987) Heart rate and cardiovascular mortality: the Framingham Study. Am Heart J 113:1489–1494
Palatini P, Benetos A, Grassi G, Julius S, Kjeldsen SE, Mancia G, Narkiewicz K, Parati G, Pessina AC, Ruilope LM, Zanchetti A, European Society of Hypertension (2006) Identification and management of the hypertensive patient with elevated heart rate: statement of a European Society of Hypertension Consensus Meeting. J Hypertens 24:603–610
Kristal-Boneh E, Silber H, Harari G, Froom P (2000) The association of resting heart rate with cardiovascular, cancer and all-cause mortality. Eight year follow-up of 3527 male Israeli employees (the CORDIS Study). Eur Heart J 21:116–124
Benetos A, Rudnichi A, Thomas F, Safar M, Guize L (1999) Influence of heart rate on mortality in a French population: role of age, gender, and blood pressure. Hypertension 33:44–52
Thomas F, Rudnichi A, Bacri AM, Bean K, Guize L, Benetos A (2001) Cardiovascular mortality in hypertensive men according to presence of associated risk factors. Hypertension 37:1256–1261
Franke J, Wolter JS, Meme L, Keppler J, Tschierschke R, Katus HA, Zugck C (2012) Optimization of pharmacotherapy in chronic heart failure: is heart rate adequately addressed? Clin Res Cardiol. PubMed PMID: 22760479
Böhm M, Borer J, Ford I, Gonzalez-Juanatey JR, Komajda M, Lopez-Sendon J, Reil JC, Swedberg K, Tavazzi L (2012) Heart rate at baseline influences the effect of ivabradine on cardiovascular outcomes in chronic heart failure: analysis from the SHIFT study. Clin Res Cardiol. PubMed PMID: 22575988
Reil JC, Custodis F, Swedberg K, Komajda M, Borer JS, Ford I, Tavazzi L, Laufs U, Böhm M (2011) Heart rate reduction in cardiovascular disease and therapy. Clin Res Cardiol 100:11–19
Fujita B, Franz M, Goebel B, Fritzenwanger M, Figulla HR, Kuethe F, Ferrari M, Jung C (2012) Prognostic relevance of heart rate at rest for survival and the quality of life in patients with dilated cardiomyopathy. Clin Res Cardiol 101:701–707
Palatini P, Thijs L, Staessen JA, Thijs L, Staessen JA, Fagard RH, Bulpitt CJ, Clement DL, de Leeuw PW, Jaaskivi M, Leonetti G, Nachev C, O’Brien ET, Parati G, Rodicio JL, Roman E, Sarti C, Tuomilehto J, Systolic Hypertension in Europe (Syst-Eur) Trial Investigators (2002) Predictive value of clinic and ambulatory heart rate for mortality in elderly subjects with systolic hypertension. Arch Intern Med 162:2313–2321
Benetos A, Thomas F, Bean KE, Pannier B, Guize L (2005) Role of modifiable risk factors in life expectancy in the elderly. J Hypertens 23:1803–1808
Palatini P, Casiglia E, Julius S, Pessina AC (1999) High heart rate: a risk factor for cardiovascular death in elderly men. Arch Intern Med 159:585–592
Singh BN (2003) Increased heart rate as a risk factor for cardiovascular disease. Eur Heart J Suppl 5:G3–G9
Magder SA (2012) The ups and downs of heart rate. Crit Care Med 40:239–245
Braunwald E (1971) Control of myocardial oxygen consumption. Am Heart J 27:416–432
Tanaka N, Nozawa T, Yasumura Y, Futaki S, Hiramori K, Suga H (1990) Heart-rate-proportional oxygen consumption for constant cardiac work in dog heart. Jpn J Physiol 40:503–521
Colin P, Ghaleh B, Monnet X, Hittinger L, Berdeaux A (2004) Effect of graded heart rate reduction with ivabradine on myocardial oxygen consumption and diastolic time in exercising dogs. J Pharmacol Exp Ther 308:236–240
Heusch G (2008) Heart rate in the pathophysiology of coronary blood flow and myocardial ischaemia: benefit from selective bradycardic agents. Br J Pharmacol 153:1589–1601
Heusch G, Yoshimoto N (1983) Effects of heart rate and perfusion pressure on segmental coronary resistances and collateral perfusion. Pflügers Arch 397:284–289
Landesberg G, Beattie WS, Mosseri M, Jaffe AS, Alpert JS (2009) Perioperative myocardial infarction. Circulation 119:2936–2944
Bassenge E, Heusch G (1990) Endothelial and neuro-humoral control of coronary blood flow in health and disease. Rev Physiol Biochem Pharmacol 116:77–165
Giannoglou GD, Chatzizisis YS, Zamboulis C, Parcharidis GE, Mikhailidis DP, Louridas GE (2008) Elevated heart rate and atherosclerosis: an overview of the pathogenetic mechanisms. Int J Cardiol 126:302–312
Soulis JV, Giannoglou GD, Chatzizisis YS, Farmakis TM, Giannakoulas GA, Parcharidis GE, Louridas GE (2006) Spatial and phasic oscillation of non-Newtonian wall shear stress in human left coronary artery bifurcation: an insight to atherogenesis. Coron Artery Dis 17:351–358
Chaniotis AK, Kaiktsis L, Katritsis D, Efstathopoulos E, Pantos I, Marmarellis V (2010) Computational study of pulsatile blood flow in prototype vessel geometries of coronary segments. Phys Med 26:140–156
Nattel S (2002) New ideas about atrial fibrillation 50 years on. Nature 415:219–226
Huang JL, Wen ZC, Chang M-S, Chen SA (1998) Changes of autonomic tone before the onset of paroxysmal atrial fibrillation. Int J Cardiol 66:275–283
Bettoni M, Zimmermann M (2002) Autonomic tone variations before the onset of paroxysmal atrial fibrillation. Circulation 105:2753–2759
Tai CT, Chiou CW, Chen SA (2002) Interaction between the autonomic nervous system and atrial tachyarrhythmias. J Cardiovasc Electrophysiol 13:83–87
Amar D, Zhang H, Miodownik S, Kadish AH (2003) Competing autonomic mechanisms precede the onset of postoperative atrial fibrillation. J Am Coll Cardiol 42:1262–1268
Lombardi F, Tarricone D, Tundo F, Colombo F, Belletti S, Fiorentini F (2004) Autonomic nervous system and paroxysmal atrial fibrillation: a study based on the analysis of RR interval changes before, during and after paroxysmal atrial fibrillation. Eur Heart J 25:1242–1248
Palatini P, Julius S (1997) Heart rate and cardiovascular risk. J Hypertens 15:3–17
Psaty BM, Manolio TA, Kuller LH, Kronmal RA, Cushman M, Fried LP, White R, Furberg CD, Rautaharju PM (1997) Incidence of and risk factors for atrial fibrillation in older adults. Circulation 96:2455–2461
Tsao HM, Yu WC, Cheng HC, Wu MH, Tai CT, Lin WS, Ding YA, Chang MS, Chen SA (2001) Pulmonary vein dilation in patients with atrial fibrillation: detection by magnetic resonance imaging. J Cardivasc Electrophysiol 12:809–813
Herweg B, Sichrovosky T, Polosajian L, Rozenshtein A, Steinberg JS (2005) Hypertension and hypertensive heart disease are associated with increased pulmonary vein diameter. J Cardiovasc Electrophysiol 16:2–5
St. André AC, DelRossi A (2005) Hemodynamic management of patients in the first 24 hours after cardiac surgery. Crit Care Med 33:2082–2093
Segal OR, Chow AWC, Peters NS, Wyn Davies D (2010) Mechanisms that initiate ventricular tachycardia in the infarcted human heart. Heart Rhythm 7:57–64
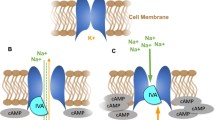
Di Francesco D (1995) The pacemaker current (If) plays an important role in regulating SA node pacemaker activity. Cardiovasc Res 30:307–308
Di Francesco D (2010) The role of the funny current in pacemaker activity. Circ Res 106:434–446
Verkek AO (2009) Pacemaker activity of the human sinoatrial node: role of the hyperpolarization-activated current, If. Int J Cardiol 132:318–336
Borer J (2008) Characterization of the heart rate-lowering action of ivabradine, a selective If current inhibitor. Am J Ther 15:461–473
Di Francesco D (2007) The funny current. Cellular basis for the control of heart rate. Drugs 67(Suppl 2):15–24
Di Francesco D (1991) Direct activation of cardiac pacemaker channels by intracellular cyclic AMP. Nature 351:145–147
Vilaine JP (2006) The discovery of the selective If current inhibitor ivabradine. A new therapeutic approach to ischemic disease. Pharmacol Res 53:424–434
Bucchi A (2007) Heart rate reduction via selective “funny” channels blockers. Curr Opin Pharmacol 7:208–213
Tardiff JC (2005) Efficacy of ivabradine, a new selective If inhibitor, compared with atenolol in patients with chronic stable angina. Eur Heart J 26:2529–2536
Fox K, Ford I, Steg PG, Tendera M, Ferrari R, BEAUTIFUL Investigators (2008) Ivabradine for patients with stable coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): a randomised, double blind, placebo-controlled trial. Lancet 372:807–816
Swedberg K, Komajda M, Böhm M, Borer J, Robertson M, Tavazzi L, Ford I; SHIFT Investigators (2012) Effects on outcomes of heart rate reduction by ivabradine in patients with congestive heart failure: is there an influence of beta-blocker dose?: Findings From the SHIFT (Systolic Heart failure treatment with the I(f) inhibitor ivabradine Trial) Study. J Am Coll Cardiol [Epub ahead of print]
Werdan K, Ebelt H, Nuding S, Höpfner F, Hack G, Müller-Werdan U (2012) Ivabradine in combination with beta-blocker improves symptoms and quality of life in patients with stable angina pectoris: results from the ADDITIONS study. Clin Res Cardiol 101:365–373
Koester R, Kaehler J, Meinertz T (2011) Ivabradine for the treatment of stable angina pectoris in octogenarians. Clin Res Cardiol 100:121–128
Koester R, Kaehler J, Ebelt H, Soeffker G, Werdan K, Meinertz T (2010) Ivabradine in combination with beta-blocker therapy for the treatment of stable angina pectoris in every day clinical practice. Clin Res Cardiol 99:665–672
Booker KJ, Holm K, Drew BJ, Lanuza DM, Hicks FD, Carrigan T, Wright M, Moran J (2003) Frequency and outcomes of transient myocardial ischemia in critically ill adults admitted for noncardiac conditions. Am J Crit Care 12:508–517
Martinez EA, Kim LJ, Faraday N, Rosenfeld BA, Bass EB, Perler BA, Williams GM, Dorman T, Pronovost PJ (2003) Sensitivity of routine intensive care unit surveillance for detecting myocardial ischemia. Crit Care Med 31:2302–2308
Marik PE (2001) Supraventricular and ventricular arrhythmias. In: Marik PE (ed) Handbook of evidence- based critical care. Springer, NewYork, pp 141–154
McCloskey DI, Mitchell JH (1972) Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol 224:173–181
Magder S (2001) Effects of respiratory muscle afferent on the breathing and the afferent hypothesis. In: Scharf SM, Pinsky MR, Magder S (eds) Respiratory–circulatory interactions in health and disease. Marcel Dekker, New York, pp 405–425
Kaufman MP, Iwamoto GA, Longhurst JC, Mitchell JH (1982) Effects of capsaicin and bradykinin on afferent fibers with endings in skeletal muscle. Circ Res 50:133–139
Relos RP, Hasinoff IK, Beilman GJ (2003) Moderately elevated serum troponin concentrations are associated with increased morbidity and mortality rates in surgical intensive care unit patients. Crit Care Med 31:2598–2603
McCann RL, Clements FM (1989) Silent myocardial ischemia in patients undergoing peripheral vascular surgery: incidence and association with perioperative cardiac morbidity and mortality. J Vasc Surg 9:583–587
Raby KE, Barry J, Creager MA, Cook EF, Weisberg MC, Goldman L (1992) Detection and significance of intraoperative and postoperative myocardial ischemia in peripheral vascular surgery. JAMA 268:222–227
Landesberg G, Luria MH, Cotev S, Eidelman LA, Anner H, Mosseri M, Schechter D, Assaf J, Erel J, Berlatzky Y (1993) Importance of long-duration postoperative ST segment depression in cardiac morbidity after vascular surgery. Lancet 341:715–719
Landesberg G, Mosseri M, Zahger D, Wolf Y, Perouansky M, Anner H, Drenger B, Hasin Y, Berlatzky Y, Weissman C (2001) Myocardial infarction after vascular surgery: the role of prolonged stress-induced, ST depression-type ischemia. J Am Coll Cardiol 37:1839–1845
Sander O, Welters ID, Foëx P, Sear JW (2005) Impact of prolonged elevated heart rate on incidence of major cardiac events in critically ill patients with a high risk of cardiac complications. Crit Care Med 33:81–88
Mangano DT, Layug EL, Wallace A, Tateo I (1996) Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery: multicenter Study of Perioperative Ischemia Research Group. N Engl J Med 335:1713–1720
Auerbach AD, Goldman L (2002) β-Blockers and reduction of cardiac events in noncardiac surgery: scientific review. JAMA 287:1435–1444
Kertai MD, Boersma E, Bax JJ, Thomson IR, Cramer MJ, van de Ven LL, Scheffer MG,Trocino G, Vigna C, Baars HF, van Urk H, Roelandt JR, Poldermans D, Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography (DECREASE) Study Group (2003) Optimizing long-term cardiac management after major vascular surgery: Role of beta-blocker therapy, clinical characteristics, and dobutamine stress echocardiography to optimize long-term cardiac management after major vascular surgery. Arch Intern Med 163:2230–2235
Devereaux PJ, Yang H, Yusuf S, Guyatt G, Leslie K, Villar JC, Xavier D, Chrolavicius S, Greenspan L, Pogue J, Pais P, Liu L, Xu S, Málaga G, Avezum A, Chan M, Montori VM, Jacka M, Choi P (2008) Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet 371:1839–1847
Vitale D, Santis V, Guarracino F, Fontana A, Pellegrini F, Tritapepe L (2010) Use of ivabradine in catecholamine-induced tachycardia after high-risk cardiac surgery. Clin Res Cardiol 99:853–855
Links A, Reil JC, Selejan S, Böhm M (2009) Effect of ivabradine in dobutamine induced sinus tachycardia in a case of acute heart failure. Clin Res Cardiol 98:513–515
Conference ACoCPoCCMC (1992) Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med 20:864–874
Marshall JC, Cook DJ, Christou NV, Bernard GR, Sprung CL, Sibbald WJ (1995) Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med 23:1638–1652
Muller-Werdan U, Buerke M, Ebelt H, Heinroth KM, Herklotz A, Loppnow H, Ruß M, Schlegel F, Schlitt A, Schmidt HB, Söffker G, Werdan K (2006) Septic cardiomyopathy- A not yet discovered cardiomyopathy? Exp Clin Cardiol 11:226–236
Zorn-Pauly K, Pelzmann B, Lang P, Mächler H, Schmidt H, Ebelt H, Werdan K, Koidl B, Muller-Werdan U (2007) Endotoxin impairs the human pacemaker current If. Shock 28:655–661
Parker MM, Shelhamer JH, Natanson C, Alling DW, Parrillo JE (1987) Serial cardiovascular variables in survivors and nonsurvivors of human septic shock: heart rate as an early predictor of prognosis. Crit Care Med 15:923–929
Hoke RS, Müller-Werdan U, Lautenschläger C, Werdan K, Ebelt H (2012) Heart rate as an independent risk factor in patients with multiple organ dysfunction: a prospective, observational study. Clin Res Cardiol 101:139–147
Hennen R, Friedrich I, Hoyer D, Nuding S, Rauchaus M, Shulze M, Schlisske S, Schwesig R, Schlitt A, Buerke M, Muller-Werdan U, Werdan K, Schmidt H (2008) Autonomic dysfunction and beta-adrenergic blockers in multiple organ dysfunction syndrome. Dtsch Med Wochenschr 133:2500–2504
Morelli A (2011) Strict heart rate control with esmolol in septic shock: a randomized, controlled, Clinical Pilot Study NCT01231698
Bollano E, Tang MS, Hjalmarson A, Waagstein F, Andersson B (2003) Different responses to dobutamine in the presence of carvedilol or metoprolol in patients with chronic heart failure. Heart 89:621–624
Tarnow J, Komar K (1988) Altered hemodynamic response to dobutamine in relation to the degree of preoperative beta-adrenoceptor blockade. Anesthesiology 68:912–919
Kindermann M, Seeland U, Ruhnke P, Böhm M, Maack C (2011) Functional effects of Β1-adrenoceptor polymorphisms on the hemodynamic response to dobutamine with and without β-blocker administration. Clin Res Cardiol 100:129–137
Borer JS (2004) Drug insight: if inhibitors as specific heart-rate reducing agents. Nat Clin Pract Cardiovasc Med 1:103–109
Manz M, Reuter M, Lauck G, Omran H, Jung W (2003) A single intravenous dose of ivabradine, a novel I(f) inhibitor, lowers heart rate but does not depress left ventricular function in patients with left ventricular dysfunction. Cardiology 100:149–155
Nuding S, Ebelt H, Hoke R, Krummenerl A, Wienke A, Muller-Werdan U, Werdan K (2011) Reducing elevated heart rate in patients with multiple organ dysfunction syndrome by the If (funny channel current) inhibitor ivabradine MODIfY Trial. Clin Res Cardiol 100:915–923
Mythen MG (2005) Postoperative gastrointestinal tract dysfunction. Anesth Analg 100:196–204
De Ferrari GM, Mazzuero A, Agnesina L, Bertoletti A, Lettino M, Campana C, Schwartz PJ, Tavazzi L (2008) Favourable effects of heart rate reduction with intravenous administration of ivabradine in patients with advanced heart failure. Eur J Heart Fail 10:550–555
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
De Santis, V., Vitale, D., Santoro, A. et al. Ivabradine: potential clinical applications in critically ill patients. Clin Res Cardiol 102, 171–178 (2013). https://doi.org/10.1007/s00392-012-0516-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-012-0516-3