Abstract
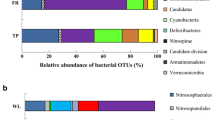
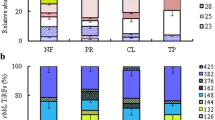
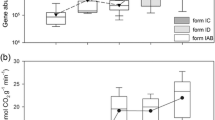
Elucidating the biodiversity of CO2-assimilating bacterial communities under different land uses is critical for establishing an integrated view of the carbon sequestration in agricultural systems. We therefore determined the abundance and diversity of CO2 assimilating bacteria using terminal restriction fragment length polymorphism and quantitative PCR of the cbbL gene (which encodes ribulose-1,5-biphosphate carboxylase/oxygenase). These analyses used agricultural soils collected from a long-term experiment (Pantang Agroecosystem) in subtropical China. Soils under three typical land uses, i.e., rice–rice (RR), upland crop (UC), and paddy rice–upland crop rotation (PU), were selected. The abundance of bacterial cbbL (0.04 to 1.25 × 108 copies g−1 soil) and 16S rDNA genes (0.05–3.00 × 1010 copies g−1 soil) were determined in these soils. They generally followed the trend RR > PU > UC. The cbbL-containing bacterial communities were dominated by facultative autotrophic bacteria such as Mycobacterium sp., Rhodopseudomonas palustris, Bradyrhizobium japonicum, Ralstonia eutropha, and Alcaligenes eutrophus. Additionally, the cbbL-containing bacterial community composition in RR soil differed from that in upland crop and paddy rice–upland crop rotations soils. Soil organic matter was the most highly statistically significant factor which positively influenced the size of the cbbL-containing population. The RR management produced the greatest abundance and diversity of cbbL-containing bacteria. These results offer new insights into the importance of microbial autotrophic CO2 fixation in soil C cycling.


Similar content being viewed by others
Abbreviations
- T-RFLP:
-
Terminal restriction fragment length polymorphism
- CO2 :
-
Carbon dioxide
- SOC:
-
Soil organic C
- RR:
-
Rice–rice
- UC:
-
Upland crop
- TRF:
-
Terminal restriction fragment
- PU:
-
Paddy rice–upland crop rotation
References
Acosta-Martíneza V, Acosta-Mercadob D, Sotomayor-Ramírezc D, Cruz-Rodríguez L (2008) Microbial communities and enzymatic activities under different management in semiarid soils. Appl Soil Ecol 38:249–260
Alfreider A, Vogt C, Hoffman D, Babel W (2003) Diversity of ribulose 1,5-bisphosphate carboxylase/oxygenase large subunit genes from groundwater and aquifer microorganisms. Microb Ecol 45:317–328
Alfreider A, Vogt C, Geiger-Kaiser M, Psenner R (2009) Distribution and diversity of autotrophic bacteria in groundwater systems based on the analysis of RubisCO genotypes. Syst Appl Microbiol 32:140–150
Badger MR, Bek EJ (2008) Multiple Rubisco forms in proteobacteria: their functional significance in relation to CO2 acquisition by the CBB cycle. J Exp Bot 59:1525–1541
Chaparro JM, Sheflin AM, Manter DK, Vivanco JM (2012) Manipulating the soil microbiome to increase soil health and plant fertility. Biol Fertil Soils 48:489–499
Chen Z, Luo XQ, Hu RG, Wu MN, Wu JS, Wei WX (2010) Impact of long-term fertilization on the composition of denitrifier communities based on nitrite reductase analyses in a paddy soil. Microb Ecol 60:850–861
Clement BG, Kehl LE, DeBord KL, Kitts CL (1998) Terminal restriction fragment patterns (TRFPs), a rapid, PCR-based method for the comparison of complex bacterial communities. J Microbiol Meth 31:135–142
Das S, Bhattacharyya P, Adhya TK (2011) Impact of elevated CO2, flooding, and temperature interaction on heterotrophic nitrogen fixation in tropical rice soils. Biol Fertil Soils 47:25–30
Elsaied H, Naganuma T (2001) Phylogenetic diversity of ribulose-1,5-bisphosphate carboxylase/oxygenase large-subunit genes from deep-sea microorganisms. Appl Environ Microbiol 67:1751–1765
Elsaied HE, Kimura H, Naganuma T (2007) Composition of archaeal, bacterial, and eukaryal RuBisCO genotypes in three Western Pacific arc hydrothermal vent systems. Extremophiles 11:191–202
Franzluebbers AJ (2010) Achieving soil organic carbon sequestration with conservation agricultural systems in the southeastern United States. Soil Sci Sco Am J 74:347–357
Hugendieck I, Meyer O (1991) Genes encoding ribulosebisphosphate carboxylase and phosphoribulokinase are duplicated in Pseudomonas carboxydovorans and conserved in carboxydotrophic bacteria. Arch Microbiol 157:92–96
Husemann M, Klintworth R, Büttcher V, Salnikow J, Weissenborn C, Bowien B (1988) Chromosomally and plasmid-encoded gene clusters for CO2 fixation (cfx) genes in Alcaligenes eutrophus. Mol Genet Genomics 214:112–120
IPCC (2007) Climate change 2007. Climate change impacts, adaptation and vulnerability. IPCC Working Group II. Geneva, Switzerland
Kaur K, Goyal S, Kapoor KK (2008) Impact of organic fertilizers with and without chemical fertilizers on soil chemical properties and the establishment of nitrogen-fixing bacteria in the rhizosphere. Microb Environ 23:313–316
Kent AD, Smith DJ, Benson BJ, Triplett EW (2003) Web-based phylogenetic assignment tool for analysis of terminal restriction fragment length polymorphism profiles of microbial communities. Appl Environ Microbiol 69:6768–6776
King GM, Weber CF (2007) Distribution, diversity and ecology of aerobic CO-oxidizing bacteria. Nat Rev Microbiol 5:107–118
Kitts CL (2001) Terminal restriction fragment patterns: a tool for comparing microbial communities and assessing community dynamics. Curr Issues Intest Microbiol 2:17–25
Kong W, Ream DC, Priscu JC, Morgan-Kiss RM (2012) Diversity and expression of RubisCO genes in a perennially ice-covered Antarctic lake during the polar night transition. Appl Environ Microbiol 78:4358–4366
Kusian B, Bowien B (1997) Organization and regulation of cbb CO2 assimilation genes in autotrophic bacteria. FEMS Microbiol Rev 21:135–155
López-Gutiérrez JC, Henry S, Hallet S, Martin-Laurent F, Catroux G, Philippot L (2004) Quantification of a novel group of nitrate-reducing bacteria in the environment by real-time PCR. J Microbiol Meth 57:399–407
Lukow T, Dunfield PF, Liesack W (2000) Use of the T-RFLP technique to assess partial and temporal changes in the bacterial community structure with in an agricultural soil planted with transgenic and nontransgenic potato plants. FEMS Microbiol Ecol 32:241–247
Marsh TL, Saxman P, Cole J, Tiedje J (2000) Terminal restriction fragment length polymorphism analysis program, a web-based research tool for microbial community analysis. Appl Environ Microbiol 66:3616–3620
Meyer O, Schlegel HG (1983) Biology of aerobic carbon monoxide oxidizing bacteria. Annu Rev Microbiol 37:277–310
Meyer O, Frunzke K, Gadkari D, Jacobitz S, Hugendieck I, Kraut M (1990) Utilization of carbon monoxide by aerobes: recent advances. FEMS Microbiol Rev 87:253–260
Midgley GF, Bond WJ, Kapos V, Ravilious C, Scharlemann JPW, Woodward F (2010) Terrestrial carbon stocks and biodiversity: key knowledge gaps and some policy implications. Curr Opin Environ Sustain 2:264–270
Nanba K, King GM, Dunfield K (2004) Analysis of facultative lithotroph distribution and diversity on volcanic deposits by use of the large subunit of ribulose 1,5-bisphosphate carboxylase/oxygenase. Appl Environ Microbiol 70:2245–2253
Nelson DW, Sommers LE (1996) Total carbon, organic carbon and organic matter. In: Sparks DL, Page AL, Helmke PA, Loeppert RH, Soltanpour PN, Tabatabai MA, CT Jh, Sumner ME (eds) Methods of soil analysis. Part 3—chemical methods. Soil Science Society of America, Madison, pp 961–1010
Okano Y, Hristova KR, Leutenegger CM, Jackson LE, Denison FR, Gebreyesus B, Lebauer D, Scow KM (2004) Application of real-time PCR to study effects of ammonium on population size of ammonia-oxidizing bacteria in soil. Appl Environ Microbiol 70:1008–1016
Osborn AM, Moore ERB, Timmis KN (2000) An evaluation of terminal restriction fragment length polymorphism (T-RFLP) analysis for the study of microbial community structure and dynamics. Environ Microbiol 2:39–50
Paul J, Alfreider A, Wawrik B (2000) Micro and macro diversity in rbcL sequences in ambient phytoplankton populations from the southeastern Gulf of Mexico. Mar Ecol Prog Ser 198:9–18
Salles JF, Van Veen JA, Van Elsas JD (2004) Multivariate analyses of Burkholderia species in soil: effect of crop and land use history. Appl Environ Microbiol 70:4012–4020
Selesi D, Schmid M, Hartmann A (2005) Diversity of green-like and red-like ribulose 1.5-bisphosphatecarboxylase/oxygenase large-subunit genes (cbbL) in differently managed agricultural soils. Appl Environ Microbiol l71:175–184
Selesi D, Pattis I, Schmid M, Kandeler E, Hartmann A (2007) Quantification of bacterial RubisCO genes in soils by cbbL targeted real-time PCR. J Microbiol Methods 69:497–503
Shannon CE (2001) A mathematical theory of communication. ACM SIGMOBILE Mob Comp Comm Rev 5(1):3–55
Shively JM, English RS, Baker SH, Cannon GC (2001) Carbon cycling: the prokaryotic contribution. Curr Opin Microbiol 4:301–306
Singleton DR, Furlong MA, Rathbun SL, Whitman WB (2001) Quantitative comparisons of 16S rRNA gene sequence libraries from environmental samples. Appl Environ Microbiol 67:4374–4376
Sombrero A, de Benitoa A (2010) Carbon accumulation in soil. Ten-year study of conservation tillage and crop rotation in a semi-arid area of Castile-Leon, Spain. Soil Tillage Res 107:64–70
Tabita FR (1999) Microbial ribulose-1,5-bisphosphatecarboxylase/oxygenase: a different perspective. Photosynth Rev 60:1–28
Tabita FR, Hanson TE, Satagopan S, Witte BH, Kreel NE (2008) Phylogenetic and evolutionary relationships of RubisCO and the RubisCO-like proteins and the functional lessons provided by diverse molecular forms. Philos Trans R Soc Lond B Biol Sci 363:2629–2640
Tolli J, King GM (2005) Diversity and structure of bacterial chemolithotrophic communities in pine forest and agroecosystem soils. Appl Environ Microbiol 71:8411–8418
Torsvik V, Øvreås L (2002) Microbial diversity and function in soil: from genes to ecosystems. Curr Opin Microbiol 5:240–245
Turova TP, Spiridonova EM (2009) Phylogeny and evolution of the ribulose 1,5-bisphosphate carboxylase/oxygenase genes in prokaryotes. Mol Biol 43:713–728
van der Wielen PWJJ (2006) Diversity of ribulose-1,5-bisphosphate carboxylase/oxygenase large-subunit genes in the MgCl2-dominated deep hypersaline anoxic basin discovery. FEMS Microbiol Lett 259:326–331
Vance ED, Brookes PC, Jenkinson DS (1987) Microbial biomass measurements in forest soils: determination of kc values and tests of hypotheses to explain the failure of the chloroform fumigation–incubation method in acid soils. Soil Biol Biochem 19:689–696
Videmšek U, Hagn A, Suhadolc M, Radl V, Knicker H, Schloter M, Vodnik D (2009) Abundance and diversity of CO2-fixing bacteria in grassland soils close to natural carbon dioxide springs. Microb Ecol 58:1–9
Witte B, John D, Wawrik B, Paul JH, Dayan D, Tabita F (2010) Functional prokaryotic RubisCO from an oceanic metagenomic library. Appl Environ Microbiol 76:2997–3003
Wu J, Joergensen RG, Pommerening B, Chaussod R, Brookes PC (1990) Measurement of soil microbial biomass C by fumigation–extraction—an automated procedure. Soil Biol Biochem 22:1167–1169
Xu HH, Tabita FR (1996) Ribulose-1,5-bisphosphatecarboxylase/oxygenasegene expression and diversity of Lake Erie planktonic microorganisms. Appl Environ Microbiol 62:1913–1921
Yao H, He Z, Wilson MJ, Campbell CD (2000) Microbial biomass and community structure in a sequence of soils with increasing fertility and changing land use. Microb Ecol 40:223–237
Yousuf B, Sanadhya P, Keshri J, Jha B (2012) Comparative molecular analysis of chemolithoautotrophic bacterial diversity and community structure from coastal saline soils, Gujarat, India. BMC Microbiol 12:150–164
Yuan HZ, Ge TD, Chen CY, O’Donnell AG, Wu JS (2012a) Microbial autotrophy plays a significant role in the sequestration of soil carbon. Appl Environ Microbiol 78:2328–2336
Yuan HZ, Ge TD, Wu XH, Liu SL, Tong CL, Qin HL, Wu MN, Wei WX, Wu JS (2012b) Long-term field fertilization alters the div ersity of autotrophic bacteria based on the ribulose-1,5-biphosphate carboxylase/oxygenase (RubisCO) large-subunit genes in paddy soil. Appl Microbiol Biotechnol 95:1061–1071
Acknowledgments
This work was supported by the “Strategic Priority Research Program—Climate Change: Carbon Budget and Related Issues” of the Chinese Academy of Sciences (XDA05050505), National Natural Science Foundation of China (41090283; 41271279), the CAS/SAFEA International Partnership Program for Creative Research Teams (KZCX2-YW-T07; 20100491005-8) and the Knowledge Innovation Program of the Chinese Academy of Sciences (ISACX-LYQY-QN-1103).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yuan, H., Ge, T., Zou, S. et al. Effect of land use on the abundance and diversity of autotrophic bacteria as measured by ribulose-1,5-biphosphate carboxylase/oxygenase (RubisCO) large subunit gene abundance in soils. Biol Fertil Soils 49, 609–616 (2013). https://doi.org/10.1007/s00374-012-0750-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-012-0750-x