Abstract
Birds undergo pronounced physiological changes during the reproduction, which may be linked to their parental efforts. Examining these changes may supply information about the birds’ energy expenditure during the particular phases of breeding and help to understand birds’ decisions about their subsequent parental investments. In this study, we measured a number of variables, i.e. body mass (scaled mass index, SMI), baseline corticosterone (CORT) and prolactin (PRL) concentrations and leucocyte profile [basically heterophils-to-lymphocytes (H/L) ratio], during the prelaying, incubation and chick rearing periods in 191 little auks (Alle alle), small Arctic seabirds. We expected the changes in the physiological variables to reflect the energy demands, i.e. the highest levels during chick rearing, moderate ones during incubation and the lowest ones prior to egg laying. Unexpectedly, we found SMI to be the highest during the incubation period, whereas lower and similar to each other values were recorded during the prelaying and chick rearing periods. Also, CORT unexpectedly peaked in the prelaying period, declined during incubation and remained at the incubation level during the chick rearing period. In accordance with our expectations, the PRL concentration was five times higher during the incubation than the chick rearing period and H/L ratio followed the pattern of the CORT changes. Consequently, there is no straightforward interpretation of the observed patterns of changes. Evidently, there are factors other than parental efforts per se that account for the dynamics of physiological changes. We found no sex differences in any of the variables investigated, which indicates that male and female parental investments are very similar.
Similar content being viewed by others
Introduction
Reproduction is believed to be one of the most demanding stages of the avian annual cycle (Drent and Daan 1980; Walsberg 1983), one that is associated with pronounced physiological changes. The parents need to acquire energy and nutrients to satisfy the immediate demands of reproduction such as territory defence, courtship, incubation and chick rearing. These efforts may influence the birds’ body condition, particularly when demands are increased in some way, either experimentally or as a result of deteriorating environmental conditions (Nur 1984; Hõrak et al. 1998; Kitaysky et al. 1999; Velando and Alonso-Alvarez 2003; Norte et al. 2009; Harding et al. 2011). Despite this, however, breeding adults undergo modifications in body condition related to reproductive physiology per se. Concentrations of various hormones change distinctly during the breeding period; this is associated with particular reproductive activities (e.g. Lormée et al. 2003; Williams 2005; Williams et al. 2008; Riou et al. 2010; Rector et al. 2012; Angelier et al. 2013). These hormonal changes have often been reported to coincide with changes in body mass (O’Dwyer et al. 2006; Groscolas et al. 2008; reviewed in Bokony et al. 2009). Furthermore, fluctuations in many hormones appear to mediate changes in immune activity (Sapolsky 1992; Skwarło-Sońta 1992; Olsen and Kovacs 1996; Råberg et al. 1998; Roberts et al. 2004; Martin et al. 2008).
In this study, we investigated the seasonal dynamics of some physiological variables and their mutual relationships in a small (140–180 g) seabird, the little auk (Alle alle) (Fig. 1), which nests in rock crevices on mountain slopes in the severe Arctic environment. The species is believed to have the highest mass-specific metabolic rate of all seabirds (Gabrielsen et al. 1991; Konarzewski et al. 1993). To satiate such high energy demands during the breeding season, these birds need to forage on specific, energy-rich prey which they obtain at distant foraging grounds (median 30–60 km, but sometimes up to 150 km from the breeding colony; Jakubas et al. 2012, 2013). Such reproductive demands are expected to affect the birds’ body condition. Both parents share to an equal extent the 28-day incubation (Wojczulanis-Jakubas et al. 2009a), brood the chick (for the first few days after hatching) and deliver food to it with similar frequency (Harding et al. 2004; Wojczulanis-Jakubas et al. 2012). Only at the end of the chick rearing period (ca 26 d, Wojczulanis-Jakubas and Jakubas 2012) does the female desert the brood, while the male continues feeding and escorts the fledgling during its first flight to sea. It has been hypothesised that female brood desertion is related to her deteriorating body condition (Harding et al. 2004). But how that could be the case, given the equal male and female contribution to parental care up to the moment of desertion, is unclear. It is possible that the costs of specific parental activities are different for males and females owing to the basic anatomical, physiological and behavioural differences between the sexes (e.g. Elliot et al. 2010). Although this issue has already been addressed, a limited range of physiological variables, never covering the whole breeding season, were examined (Jakubas et al. 2008; Wojczulanis-Jakubas et al. 2012; Wojczulanis-Jakubas and Jakubas 2012).
For this, we used the following physiological variables: body mass corrected for body size (BMI), baseline concentration of corticosterone (CORT) and prolactin (PRL) and leucocyte profile. Body mass, if appropriately corrected for body size, is a valuable and commonly used estimate of body condition (e.g. Moe et al. 2002; Lormée et al. 2003; Rector et al. 2012; Elliott et al. 2014). The body mass of an individual bird has been reported to change in response to its increased efforts, including those associated with reproduction (e.g. Kitaysky et al. 1999; Norte et al. 2009; Harding et al. 2011). The two hormones focused on here have been widely investigated in the context of the reproductive performance. A moderate elevation of baseline CORT can help fuel sustained challenging activities such as chick brooding and provisioning (Landys et al. 2006). However, when CORT level reaches a threshold, it acts to shift energy away from reproduction towards survival (Angelier et al. 2009). PRL is elevated during the period of parental care (Buntin 1996), and this is well reflected in differences in the level of circulating PRL in species of birds performing various parental behaviours (Van Roo et al. 2003; Boos et al. 2007; Angelier and Chastel 2009). The secretion of PRL may be regulated through internal mechanisms or through tactile stimuli (Goldsmith 1991). Correspondingly, the level may be elevated during the whole period of parental care (e.g. Vleck et al. 2000) or may drop abruptly soon after the interruption of incubation and/or brooding (Opel and Proudman 1988; Richard-Yris et al. 1998). Hence, monitoring hormone concentrations throughout the breeding season enables one to establish the extent of the birds’ involvement in parental duties. As the secretion of both hormones has been found to affect the immune system (Yu-Lee 2002; Roberts et al. 2007), we also examined the leucocyte profile, which provides a convenient measure of integrated immune function. In particular, the ratio of the two most common leucocytes in birds [heterophils to lymphocytes (H/L)] is often used as an avian stress indicator. H/L ratio rises during challenging situations, and it is positively correlated with the baseline CORT level (reviewed in Davis et al. 2008).
By studying physiological variables in the little auk, we aimed to characterise a general pattern of changes throughout the breeding season in this species. In addition, we discuss the pattern observed in the context of the species-specific traits of its breeding biology. Specifically, we expected the highest (BMI) or lowest values (CORT, H/L) of the variables, indicative of the greatest investments, during the chick rearing period, when the parents need to perform frequent foraging trips to provision the rapidly developing chick (see also Elliott et al. 2014). Then, we expected moderate ranges of the variables during the incubation period, when the main parental activity is warming the egg. Finally, we expected the lowest (CORT, H/L) or highest (BMI) values of the variables, indicating the lowest energy expenditures, during the prelaying period, when the breeding birds are not yet burdened with any parental duties. PRL was only examined during the incubation and chick rearing periods, and we expected its level to be higher during the incubation period. With respect to sex differences, in the light of the hypothesis presented above, we expected females to have a lower BMI, and higher CORT, PRL and H/L levels than males, particularly during the chick rearing period. Finally, having measured several variables simultaneously, we investigated their mutual relationships in order to acquire insight into the potential influence of the variables on each other.
Materials and methods
Study area and field work
We carried out the study in the large little auk breeding colony on the Ariekammen slopes in Hornsund (SW Spitsbergen; 77°00′N, 15°33′E). We captured the birds three times during the breeding season, i.e. during the prelaying, incubation and chick rearing periods, using noose carpets deployed over the colony area, or by hand, directly in the nest while the birds were incubating. We captured birds over 4–5 days during each breeding period. The timing of capturing lay within 9–18 day prior to egg laying from day 2 to 17 of the incubation period and from day 11 to 20 of the chick rearing period. We established the birds’ phenology based on the exact egg laying dates of the incubating birds and the median egg laying and hatching dates in the colony for the birds captured in the prelaying and chick rearing periods. To establish the median dates, we monitored 112 and 169 nests daily prior egg laying and hatching, respectively. The precise synchrony of egg laying (within 5 days) and hatching (80 % of nests hatched within 7 days) in the monitored nests allowed us to assume a similar stage of breeding for all the individuals captured during the non-incubation periods.
We took only breeding adults into consideration. To establish the breeding status of individuals captured during the prelaying period, we marked the birds with a unique colour sign on the breast feathers and observed them for a few consecutive days during the prelaying period (also for the purposes of another study, Wojczulanis-Jakubas et al. 2014a). Birds copulating regularly were considered to be breeders. The breeding status of incubating birds was self-evident, while gular pouches full of food in the birds captured during the chick rearing period indicated chick provisioning.
From every captured bird, we collected a blood sample from its brachial vein in a 200-µL heparinised capillary. Since the stress associated with the handling procedure is known to affect the baseline level of hormones, we collected the samples within 3 min of capture, as recommended in the literature (Romero and Reed 2005). To avoid repeat sampling of the same individual (and hence the problem of pseudoreplication), we individually ringed the sampled birds. The collected blood was kept cool in the field (+4 °C) for 2–3 h until division of the sample for doing blood smears, hormonal analyses (from plasma) and molecular sexing (from red blood cells). The blood samples were gently stirred to combine fractions that could have separated before the smear was made with ca 5 µL of the blood and the rest of the sample centrifuged for 10 min at 2000×g. The plasma and red blood cells were separately kept frozen (at −20 °C) and analysed within 4 months. We also weighed (with an electronic balance accurate to 0.1 g) and measured (overall head-bill length, from the most distant point of the occipital to the tip of the upper mandible, with a calliper accurate to 0.01 mm) each bird sampled.
In total, we sampled 191 birds, including 36 males and 38 females during the prelaying period, 28 males and 28 females during incubation, and 34 males and 27 females during the chick rearing period. However, the amount of the blood collected from some birds was not sufficient to perform all the analyses, and the quality of some smears was not good enough to reliably assess the leucocyte profile. Therefore, certain variables relating to the sample sizes given in the results section vary slightly. Also, the PRL concentration was measured only during the incubation and chick rearing periods, as this is the hormone associated exclusively with parental care (Buntin 1996).
We released all the birds or returned them directly to the nest unharmed after the whole procedure. The fieldwork was carried out by kind permission of the Norwegian Animal Research Committee and the Governor of Svalbard.
Laboratory work
We measured baseline concentrations of total (free and bound) CORT and PRL at the Centre d´Etudes Biologiques de Chizé, France. We measured the total (free and bound) plasma CORT by radioimmunoassay following the procedure described in detail by Lormée et al. (2003) and plasma PRL by a heterologous radioimmunoassay (RIA) as described in Cherel et al. (1994). We ran all the samples in one assay for both hormones. To measure intra-assay variation, we included four different samples ten times in both hormone assays. The intra-assay variation for total CORT and PRL levels was within the 5–12 % range. The method for both hormones was previously validated in the little auk and described in detail in Wojczulanis-Jakubas et al. (2013).
We stained the blood smears using the May–Grünewald–Giemsa method (Lillie 1977) with the Wescor “Aerospray Hematology” stainer. We examined blood cells by 400–1000× microscopy. We assessed the proportion of different types of leucocytes (heterophils, lymphocytes, basophils, eosinophils and monocytes) based on the examination of 100 leucocytes under an oil immersion. Because the numbers of monocytes, eosinophils and basophils were low (S1), we disregarded these leucocytes in subsequent analyses. Since H/L ratio may not always reflect the overall allocation to the leucocyte production (Lobato et al. 2005), we also counted the total number of leucocytes per 10,000 erythrocytes in a data subset (20, 14 and 20 females randomly chosen among those sampled during the prelaying (63 %, N = 38), incubation (50 %, N = 28) and chick rearing periods (80 %, N = 25), respectively. Since H/L ratio and the total number of leucocytes corresponded well, we give H/L data here, the data for the total number of leucocytes will be found in the Supplementary material (S2).
We extracted DNA for molecular sexing from the frozen red blood cells using a Blood Mini Kit (A&A Biotechnology, Gdynia, Poland). We performed the CHD gene-based analysis with the primer pair F2550 and R2718, according to Griffiths et al. (1998), using a 50 °C annealing temperature for the PCR. The sex differences in the PCR products were clearly visible in UV light, when the fragments were separated on 2 % agarose gel stained with ethidium bromide.
Statistical analyses
A great many methods for correcting body mass for body size have been put forward (e.g. Albrecht et al. 1993; García-Berthou 2001; Schulte-Holstedde et al. 2005; Peig and Green 2009). Since there is no agreement about which one performs this correction in the most efficient way, we tested four different methods, all with overall head-bill length as surrogate measure of body size. We used this length as it is considered to be a representative measurement of the little auk’s structural size (Jakubas and Wojczulanis 2007). Firstly, we corrected the body mass with the body condition index (BCI) sensu Albrecht et al. (1993). We computed it using the formula: BCI = M/L, where M is the body mass and L is the linear body measurement of an individual (Albrecht et al. 1993). Secondly, we corrected body mass using the residuals of a simple linear regression of body mass and linear body measurements following the recommendations of Schulte-Holstedde et al. (2005). Thirdly, we performed analysis of covariance (ANCOVA) with the linear body measurement being a covariate (García-Berthou 2001). Finally, we calculated the scaled mass index proposed by Peig and Green (2009). All these methods delivered qualitatively similar results in terms of the seasonal changes but differed in efficiency of the body mass to body size correction, which, in turn, affected the sex difference issue. Here, we present the results obtained using the method of Peig and Green (2009), which efficiently corrected body mass for body size. The results of the analyses performed with the other methods of body mass correction are given in the Supplementary material (S3).
To adjust a bird’s body mass (BMI) with the scaled mass index (SMI), we used the following formula (Peig and Green 2009):
where M i is the body mass of an individual i, L i is the linear body measurement of an individual i (overall head-bill length) and b SMA is the scaling exponent estimated from the regression of M and L. We calculated the scaling exponent by dividing the slope of the ordinary linear square regression of lnM and lnL by Pearson’s correlation coefficient (LaBarbera 1989; Peig and Green 2009). L o is the arithmetic mean value of the linear measurement. We used the mean value of overall head-bill length for the set of birds examined.
We checked the assumptions of normality and homogeneity of variance of all the variables using the Shapiro–Wilk and Leven tests, respectively. As we did not find any indications that the assumptions had been violated, we used the parametric tests. To examine the changes in the physiological measures of males and females throughout the breeding season, we performed a separate factorial ANOVA for each variable (i.e. SMI, CORT, PRL, H/L), with breeding stage and the sex of birds included as fixed factors. In all models, we included the breeding stage × sex interaction and used sums of squares of type III. We used the Newman–Keuls test to further examine significant differences revealed by factorial ANOVA. We presented the values as means and 95 % confidence intervals. We analysed the relationships between each two physiological variables with Pearson’s correlation, using data for both sexes and all breeding stages combined. We performed all the analyses in STATISTICA 9.0 (StatSoft Inc. 2010).
Results
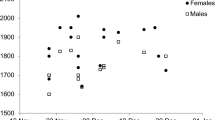
The scaled mass index (SMI) varied across the breeding season (ANOVA, F 2,189 = 8.94, P < 0.001, Fig. 2). The highest values of SMI were recorded during the incubation period, although this was statistically significant only when this stage was compared with to the chick rearing period (Newman–Keuls tests, for both sexes P < 0.02, Fig. 2). Also, the values of SMI were similar during the prelaying and chick rearing periods (Newman–Keuls tests, for both sexes P > 0.15, Fig. 2). Neither sex (F 1,189 = 3.35, P = 0.07, Fig. 2) nor the breeding stage × sex interaction (ANOVA, F 2,189 = 0.01, P = 0.99) affected SMI significantly.
Body mass (corrected for body size using scaled mass index, SMI; means and 95 % confidence intervals) of the little auk (Alle alle) males and females during the successive periods of the breeding season. The numbers below and above the bars indicate the sample sizes for males and females, respectively
The baseline CORT concentration differed between breeding stages (ANOVA, F 2,156 = 14.73, P < 0.001) but not between sexes (F 1,156 = 0.14, P = 0.71). The highest CORT concentration reached its highest level during the prelaying period. A significant difference was found when the prelaying period was compared with the incubation and chick rearing periods (Newman–Keuls tests, P < 0.001; Fig. 3a). The breeding stage × sex interaction was not significant (ANOVA, F 2,156 = 0.21, P = 0.30). The level of PRL was ca five times higher during the incubation than during the chick rearing period (ANOVA, F 1,69 = 487.07, P < 0.001), but there were no differences between sexes (F 1, 69 = 2.46, P = 0.12; Fig. 3b). The breeding stage × sex interaction was not significant here either (ANOVA, F 1,69 = 0.59, P = 0.44).
Baseline concentrations of a corticosterone (CORT) and b prolactin (PRL) (means and 95 % confidence intervals) in the little auk (Alle alle) males and females during the successive periods of the breeding season. Numbers below and above the bars indicate the sample sizes for males and females, respectively
The heterophils-to-lymphocytes (H/L) ratios differed between the breeding stages (ANOVA, F 2,184 = 426.06, P < 0.001) but not between sexes (F 1,184 = 1.96, P = 0.20). The H/L ratios were the highest during the prelaying period; the values recorded during the incubation and chick rearing periods were significantly lower (Newman–Keuls tests, for all P < 0.001; Fig. 4). The breeding stage × sex interaction was not significant (ANOVA, F 2,184 = 0.11, P = 0.89). The changes in the H/L ratio were caused by the opposite direction of changes in the relative numbers of heterophils and lymphocytes (Fig. 4).
Heterophils-to-lymphocytes (H/L) ratio and the relative number of heterophils and lymphocytes (means and 95 % confidence intervals) in the little auk (Alle alle) males and females during the successive periods of the breeding season. The numbers below and above the bars indicate the sample sizes for males and females, respectively
SMI was significantly correlated with the CORT (negatively) and PRL (positively) concentrations (Fig. 5). In addition, the H/L ratio was significantly and positively correlated with the two hormone concentrations (Fig. 5). No significant relationships were found between SMI and the H/L ratio, or between the two hormone concentrations (Fig. 5).
Relationships between particular body condition variables in the little auk (Alle alle) at all stages of the breeding season combined (results of Pearson correlations presented). Significant correlations are denoted by bold arrows. Individual variation is presented for significant correlations in the relevant corner panels
Discussion
As expected for the SMI of the breeding little auks, the values of this index were significantly lower during the chick rearing period, when birds were brooding, and searching for, collecting and delivering the food to their offspring (Harding et al. 2004; Wojczulanis-Jakubas et al. 2012), than during the incubation period, when they mainly incubated the egg and spent the remaining time resting and foraging for themselves (Wojczulanis-Jakubas et al. 2009a). Obviously, the activities during the chick rearing period may be energetically more expensive than during incubation (Reid et al. 2002; Elliott et al. 2014). Surprisingly, however, the SMIs of chick rearing adults were similar to those measured prior to egg laying. This may suggest that the birds’ behaviours during these two breeding periods, though entirely different in nature, are similar in terms of energy expenditures. In fact, the prelaying period is usually neglected in the calculation of breeding expenses, whereas there is a growing body of evidence indicating that energy- and time-consuming behaviours are performed at that time (territory defence and mate guarding in males, egg production in females, Lormée et al. 2003; Williams 2005; Jakubas et al. 2008; Wojczulanis-Jakubas et al. 2009b; 2014a, b). It is also possible that the changes in little auk body mass were related to fluctuations in the PRL level. We found a significant and positive relationship between SMI and PRL and that is consistent with other studies showing significant relationship between PRL concentration and body mass (e.g. O’Dwyer et al. 2006). Although these are only correlational studies, they suggest a functional link between PRL and body mass. In fact, PRL has been found to increase food intake in birds (Buntin et al. 1999). Thus, a high PRL concentration in incubating little auks could stimulate them to forage intensively. Then, the fivefold drop of PRL level during the chick rearing period, associated with the interruption of incubation and brooding (Opel and Proudman 1988; Richard-Yris et al. 1998), could trigger body mass loss (Croll et al. 1991; Jones 1998). It has been hypothesised that such a loss of body mass during the most energetically demanding period of breeding may be programmed to ensure a lower cost of foraging through lowered wing load, which decreases the costs of flight and/or diving (Freed 1981; Norberg 1981, Elliot et al. 2013). The dynamics of PRL secretion could be part of such a mechanism.
We had expected the CORT concentration to be the highest during the most energetically demanding chick rearing period (Gabrielsen et al. 1991; Konarzewski et al. 1993; Lormée et al. 2003; Love et al. 2004; Landys et al. 2006), with moderate and low values during the incubation and prelaying periods, respectively. What we found, however, was that the CORT concentration peaked during the prelaying period, declined during incubation and then remained at the incubation level during the chick rearing period. The prelaying CORT peak could be explained by the stressful behaviours displayed at this time, such as aggressive interactions associated with mate and nest-site guarding and copulations (Wojczulanis-Jakubas et al. 2009b, 2014a, b). As already mentioned, the prelaying period used to be an underestimated period in terms of energy requirements and stress levels (Lormée et al. 2003; Williams 2005; Jakubas et al. 2008; Wojczulanis-Jakubas et al. 2009b; 2014a, b). The low values of CORT during the most energetically demanding chick rearing period are more difficult to explain. One possibility is that the CORT level at that period might be somehow suppressed, as such a lower level might be adaptive. A chronically elevated level of the hormone might directly affect the birds’ survival (Goutte et al. 2010), so the parents would benefit by a lowered baseline CORT concentration. Even so, such a low CORT level during the chick rearing period was apparently still high enough to guarantee effective chick provisioning under the given foraging conditions. It could also be easily elevated if the trophic situation at the foraging grounds deteriorated (Welcker et al. 2009; Kidawa et al. 2014). Perhaps, the trend of changes would have been somewhat different if we had measured corticosterone binding globulin, which is considered to be a better estimator of stress level (Love et al. 2004; Breuner et al. 2013). Also, as this is a single-season study, and given the possibility of CORT levels changing in response to foraging conditions (Welcker et al. 2009; Kidawa et al. 2014), we are unable to say to what extent the observed pattern of CORT changes in the little auk is universal. Different seasonal patterns of CORT changes across colonies and years have been observed in other seabirds (Buck et al. 2007), including closely related common guillemots (Uria aalge) (Kitaysky et al. 2007). However, it is possible that a pattern of high CORT levels during the prelaying period with a marked decrease during incubation and chick rearing is typical for cavity-nesting alcids, as similar patterns have been observed in two other burrow-nesting species, the tufted puffin (Fratercula cirrhata) (Williams et al. 2008) and Atlantic puffin (F. arctica) (Rector et al. 2012). This may be related to the safety of the burrow, which provides a protection against predators and the aggression of conspecifics. In such conditions, the chick rearing period may be extended, and so the parental birds may be more relaxed.
The PRL level was at its highest value during incubation and then decreased fivefold during the chick rearing phase, which was consistent with our expectations. Secretion of this hormone is believed to be triggered by tactile contact between the eggs and the brood patch in the precocial species (Opel and Proudman 1988; Richard-Yris et al. 1998). In the little auk, which raises a semi-precocial chick, similar mechanisms may be related to PRL secretion. One cannot rule out the possibility, however, that PRL levels in the little auk are regulated internally as, due to foraging constraints, the birds may stay away from the nest too long to maintain the hormone concentration at the appropriate level (Vleck et al. 2000). If so, it could also be a part of the mechanism responsible for the hypothesised programmed loss of body mass (see above).
We expected the pattern of changes in H/L ratio to be similar to the pattern of CORT dynamics, and this is exactly what we found. Owing to the fact that we measured immune variables of the little auk in the parasite-/pathogen-scarce Arctic ecosystem (Bennet et al. 1992), we can interpret the observed changes in the H/L ratio as being driven by an internal mechanism. Some studies suggest that changes in the H/L ratio are due to glucocorticoid-induced redistribution of cells from the other body compartments to the blood for heterophils and in the opposite direction for lymphocytes (Dhabhar et al. 1994; 1996). This simultaneous influx of heterophils into the blood and exodus of lymphocytes from the blood cause significant changes in the circulating numbers of these two types of leucocytes. This, in turn, may impose a differential ability to mount an immune response at the time of cell redistribution. Interestingly, we also found a positive relationship between the H/L ratio and PRL. This may be explained in two ways. Firstly, whereas the pituitary is the most important source of PRL in serum, PRL is also produced by leucocytes (Kooijman and Gerlo 2011). Hence, a greater number of leucocytes could result in an increase in PRL. Indeed, the pattern of H/L changes corresponded to the pattern of changes in the total number of leucocytes (Supplementary material, S2). Secondly, many studies of mammals indicate that PRL may be an important factor directly or indirectly influencing the immune system (Skwarło-Sońta 1992). This issue has been investigated much less in birds, but existing evidence points to immunophysiological similarities between these two groups of vertebrates (Skwarło-Sońta 1992). Clearly, more studies are needed to understand this relationship in avian systems.
It has been hypothesised that the little auk female deserts the brood because her body reserves have become depleted. Previous studies with single body condition variables at particular stages of breeding did not support this hypothesis (Jakubas et al. 2008; Wojczulanis-Jakubas et al. 2012; Wojczulanis-Jakubas and Jakubas 2012). Moreover, others studies, not necessarily testing this hypothesis but presenting data on energy expenditure in both sexes (Welcker et al. 2009), did not reveal any sex differences. Similarly, the present results, with a wide spectrum of body condition variables examined throughout the breeding season, do not provide evidence to support this hypothesis.
Summing up, we found pronounced changes in all the body condition variables that we monitored in the little auk throughout the breeding season. The observed patterns seem to be only partly linked to the adequate parental efforts. This indicates that there are factors other than parental efforts per se that account for the dynamics of physiological changes. We did not find any sex differences in any physiological variable, which is indicative of very similar male and female parental investments. In this context, the hypothesis that earlier brood abandonment by female is due to body reserves depletion appears to be undermined.
References
Albrecht GH, Gelvin BR, Hartman SE (1993) Ratios as a size adjustment in morphometrics. Am J Phys Anthropol 91:441–468
Angelier F, Chastel O (2009) Stress, prolactin and parental investment in birds: a review. Gen Comp Endocrinol 163:142–148
Angelier F, Clément-Chastel C, Welcker J, Gabrielsen GW, Chastel O (2009) How does corticosterone affect parental behaviour and reproductive success? A study of prolactin in black-legged kittiwakes. Funct Ecol 23:784–793
Angelier F, Wingfield JC, Trouve C, de Grissac S, Chastel O (2013) Modulation of the prolactin and the corticosterone stress responses: Do they tell the same story in a long-lived bird, the Cape petrel? Gen Comp Endocrinol 182:7–15
Bennet GF, Montgomerie R, Seutin G (1992) Scarcity of hematozoa in birds breeding on the Arctic tundra of North America. Condor 94:289–292
Bokony V, Lendvai AZ, Liker A, Angelier F, Wingfield JC, Chastel O (2009) Stress response and the value of reproduction: Are birds prudent parents? Am Nat 173:589–598
Boos M, Zimmer C, Carriere A, Robin JP, Petit O (2007) Post-hatching parental care behaviour and hormonal status in a precocial bird. Behav Process 76:206–214
Breuner CW, Delehanty B, Boonstra R (2013) Evaluating stress in natural populations of vertebrates: total CORT is not good enough. Funct Ecol 27:24–36
Buck CL, O’Reilly KM, Kildaw SD (2007) Interanual variability of Black-legged Kittiwake productivity is reflected in baseline plasma corticosterone. Gen Comp Endocrinol 150:430–436
Buntin JD (1996) Neural and hormonal control of parental behavior in birds. Adv Study Behav 25:161–213
Buntin JD, Hnasko RM, Zuzick PH (1999) Role of the ventromedial hypothalamus in prolactin-induced hyperphagia in ring doves. Physiol Behav 66:255–261
Cherel Y, Mauget R, Lacroix A, Gilles J (1994) Seasonal and fasting related changes in circulational gonadal steroids and prolactin in king penguins, Aptenodytes patagonicus. Physiol Biochem Zool 67:1154–1173
Croll DA, Gaston AJ, Noble DG (1991) Adaptive mass loss in thick-billed murres. Condor 93:496–502
Davis AK, Maney DL, Maerz JC (2008) The use of leukocyte profiles to measure stress in vertebrates: a review for ecologists. Funct Ecol 22:760–772
Dhabhar FS, Miller AH, Stein M, McEwen BS, Spencer RL (1994) Diurnal and stress-induced changes in distribution of peripheral blood leukocyte subpopulations. Brain Behav Immun 8:66–79
Dhabhar FS, Miller AH, McEwen BS, Spencer RL (1996) Stress-induced changes in blood leukocyte distribution—role of adrenal steroid hormones. J Immunol 157:1638–1644
Drent RH, Daan S (1980) The prudent parent: energetic adjustments in avian breeding. Ardea 68:225–252
Elliot KE, Gaston AJ, Crump D (2010) Sex-specific behaviour by monomorphic seabird represents risk partitioning. Behav Ecol 21:1024–1032
Elliot HE, Ricklefs RE, Gaston AJ, Hatch SA, Speakman AR, Davoren GK (2013) High flight costs, but low dive costs, in auks support the biomechanical hypothesis fir flightlessness in penguins. Proc Nat Sci Acad 110:9380–9384
Elliott KH, Le Vaillant M, Kato A, Gaston AJ, Ropert-Coudert Y, Hare JF, Speakman JR, Croll D (2014) Age-related variation in energy expenditure in a long-lived bird within the envelope of an energy ceiling. J Anim Ecol 83:136–146
Freed LA (1981) Loss of mass in breeding wrens: stress or adaptation. Ecology 62:1179–1186
Gabrielsen GW, Taylor JRE, Konarzewski M, Mehlum M (1991) Field and laboratory metabolism and thermoregulation in dovekies (Alle alle). Auk 108:71–78
García-Berthou E (2001) On the misuse of residuals in ecology: testing regression residuals vs. the analysis of covariance. J Anim Ecol 70:708–711
Goldsmith AR (1991) Prolactin and avian reproductive strategies. Acta Congr Int Ornithol 4:2063–2071
Goutte A, Angelier F, Welcker J, Moe B, Clément-Chastel C, Gabrielsen GW, Bech C, Chastel O (2010) Long-term survival effect of corticosterone manipulation in black-legged kittiwakes. Gen Comp Endocrnol 167:246–251
Griffiths R, Double MC, Orr K, Dawson RJG (1998) A DNA test to sex most birds. Mol Ecol 7:1071–1075
Groscolas R, Lacroix A, Robin JP (2008) Spontaneous egg or chick abandonment in energy-depleted king penguins: A role for corticosterone and prolactin? Horm Behav 53:51–60
Harding AMA, Van Pelt TI, Lifjeld JT, Mehlum F (2004) Sex differences in Little Auk Alle alle parental care: transition from biparental to paternal-only care. Ibis 146:642–651
Harding AMA, Welcker J, Steen H, Hamer KC, Kitaysky AS, Fort J, Talbot SL, Cornick LA, Karnovsky NJ, Gabrielsen GW, Grémillet D (2011) Adverse foraging conditions may impact body mass and survival of a high Arctic seabird. Oecologia 167:49–59
Hõrak P, Ots I, Murumägi A (1998) Haematological health state indices of reproducing Great Tits: a response to brood size manipulation. Funct Ecol 12:750–756
Jakubas D, Wojczulanis K (2007) Predicting the sex of dovekies by discriminant analysis. Waterbirds 30:92–96
Jakubas D, Wojczulanis-Jakubas K, Kreft R (2008) Sex differences in body condition and hematological parameters in little auk Alle alle during the incubation period. Ornis Fenn 85:90–97
Jakubas D, Iliszko L, Wojczulanis-Jakubas K, Stempniewicz L (2012) Foraging by little auks in the distant marginal sea ice zone during the chick-rearing period. Polar Biol 35:73–81
Jakubas D, Trudnowska E, Wojczulanis-Jakubas K, Iliszko L, Kidawa D, Darecki M, Błachowiak-Samołyk K, Stempniewicz L (2013) Foraging closer to the colony leads to faster growth in little auks. Mar Ecol Prog Ser 489:263–278
Jones IL (1998) Mass changes of least auklets Aethia pusilla during the breeding season: evidence for programmed loss of mass. J Anim Ecol 63:71–78
Kidawa D, Wojczulanis-Jakubas K, Jakubas D, Palme R, Stempniewicz L, Barcikowski M, Keslinka-Nawrot L (2014) Variation in faecal corticosterone metabolites in an Arctic seabird, the little auk (Alle alle) during the nesting period. Polar Biol 37:641–649
Kitaysky AS, Wingfield JC, Piatt JF (1999) Dynamics of food availability, body condition and physiological response in breeding Black-legged Kittiwakes. Funct Ecol 13:577–585
Kitaysky AS, Piatt JF, Wingfield JC (2007) Stress hormones link food availability and population processes in seabirds. Mar Ecol Prog Ser 352:245–258
Konarzewski M, Taylor JRE, Gabrielsen GW (1993) Chick energy requirements and adult energy expenditures of Dovekies (Alle alle). Auk 110:603–609
Kooijman R, Gerlo S (2011) Leucocyte-drive prolactin. Adv Neuroimmune Biol 1:133–142
LaBarbera M (1989) Analyzing body size as a factor in ecology and evolution. Annu Rev Ecol Evol Syst 20:97–117
Landys MM, Ramenofsky M, Wingfield JC (2006) Actions of glucocorticoids at a seasonal baseline as compared to stress-related levels in the regulation of periodic life processes. Gen Comp Endocrinol 148:132–149
Lillie RD (1977) Conn’s Biological Stains, 9th edn. Williams and Wilkins Company, Baltimore
Lobato E, Moreno J, Merino S, Sanz JJ, Arriero E (2005) Haematological variables are good predictors of recruitment in nestling pied flycatchers (Ficedula hypoleuca). Ecoscience 12:27–34
Lormée H, Jouventin P, Trouve C, Chastel O (2003) Sex-specific patterns in baseline corticosterone and body condition changes in breeding red-footed boobies Sula sula. Ibis 145:212–219
Love OP, Breuner CW, Vezina F, Williams TD (2004) Mediation of a corticosterone-induced reproductive conflict. Horm Behav 46:59–65
Martin LB, Weil ZM, Nelson RJ (2008) Seasonal changes in vertebrate immune activity: mediation by physiological trade-offs. Phil Trans R Soc B 363:321–339
Moe B, Langseth I, Fyhn M, Gabrielsen GW, Bech C (2002) Changes in body condition in breeding kittiwakes Rissa tridactyla. J Avian Biol 33:225–234
Norberg RA (1981) Temporary weight decrease in breeding birds may result in more fledged young. Am Nat 118:838–850
Norte AC, Ramos JA, Sousa JP, Sheldon BC (2009) Variation of adult great tit parus major body condition and blood parameters in relation to sex, age, year and season. J Ornithol 150:651–660
Nur N (1984) The consequences of brood size for breeding blue tits I. adult survival, weight change and the cost of reproduction. J Anim Ecol 53:479–496
O’Dwyer TW, Buttemer WA, Priddel DM, Dowining JA (2006) Prolactin, body condition and the cost of good parenting: an inter-year study in a long-lived seabird, Gould’s Petrel (Pterodroma leucoptera). Funct Ecol 20:806–811
Olsen NJ, Kovacs WJ (1996) Gonadal steroids and immunity. Endocr Rev 17:369–384
Opel H, Proudman JA (1988) Effects of poults on plasma concentrations of prolactin in turkey hens incubating without eggs or a nest. Br Poult Sci 29:791–800
Peig J, Green AJ (2009) New perspective for estimating body condition from mass/length data: the scaled mass index as an alternative method. Oikos 118:1883–1891
Råberg L, Grahn M, Hasselquist D, Svensson E (1998) On the adaptive significance of stress-induced immunosuppression. Proc R Soc B 265:1637–1641
Rector ME, Kouwenberg A-L, Wilhelm SI, Robertson GJ, McKay DW, Fitzsimmons MG, Baker CR, Cameron-MacMillan ML, Walsh CJ, Storey AE (2012) Corticosterone levels of Atlantic puffins vary with breeding stage and sex but are not elevated in poor foraging years. Gen Comp Endocrinol 178:408–416
Reid JM, Monaghan P, Nager R (2002) Incubation and the cost of reproduction. In: Deeming DC (ed) Avian incubation, behaviour, environment and evolution. Oxford University Press, Oxford, pp 314–325
Richard-Yris MA, Sharp PJ, Wauters AM, Guémené D, Richard JP, Forasté M (1998) Influence of stimuli from chicks on behavior and concentrations of plasma prolactin and luteinizing hormone in incubating hens. Horm Behav 33:139–148
Riou S, Chastel O, Lacroix A, Hamer KC (2010) Stress and parental care: prolactin responses to acute stress throughout the breeding cycle in a long-lived bird. Gen Comp Endocrnol 168:8–13
Roberts ML, Buchanan KL, Evans MR (2004) Testing the immunocompetence handicap hypothesis: a review of the evidence. Anim Behav 68:227–239
Roberts ML, Buchanan KL, Hasselquist D, Evans MR (2007) Effects of testosterone and corticosterone on immunocompetence in the zebra finch. Horm Behav 51:126–134
Romero LM, Reed JM (2005) Collecting baseline corticosterone samples in the field: Is under 3 min good enough? Comp Biochem Physiol A: Mol Integr Physiol 140:73–79
Sapolsky RM (1992) Neuroendocrinology of the stress-response. In: Becker JB, Breedlove SM, Crews D (eds) Behavioral endocrinology. MIT Press, Cambridge, pp 287–324
Schulte-Holstedde AI, Zinner B, Mills JS, Hickling GJ (2005) Restitution of mass-size residuals: validating body condition indices. Ecology 86:155–163
Skwarło-Sońta K (1992) Prolactin as an immunoregulatory hormone in mammals and birds. Immunol Lett 33:105–121
Van Roo BL, Ketterson ED, Sharp PJ (2003) Testosterone and prolactin in two songbirds that differ in paternal care: the blue-headed vireo and the red-eyed vireo. Horm Behav 44:435–441
Velando A, Alonso-Alvarez C (2003) Differential body condition regulation by males and females in response to experimental manipulations of brood size and parental effort in the blue-footed booby. J Anim Ecol 72:846–856
Vleck C, Ross LL, Vleck D, Bucher TL (2000) Prolactin and parental behavior in Adélie Penguins: effects of absence from nest, incubation length, and nest failure. Horm Behav 38:149–158
Walsberg GE (1983) Avian ecological energetics. In: Farner DS, King JR, Parkes KC (eds) Avian biology. Academic Press, UK
Welcker J, Harding AMA, Kitaysky AS, Speakman JR, Gabrielsen GW (2009) Daily energy expenditure increases in response to low nutritional stress in an Arctic-breeding seabird with no effect on mortality. Funct Ecol 23:1081–1090
Williams TD (2005) Mechanisms underlying the costs of egg production. Bioscience 55:39–48
Williams CT, Kitaysky AS, Kettle AB, Buck L (2008) Corticosterone levels of tufted puffins vary with breeding stage, body condition index, and reproductive performance. Gen Comp Endocrinol 158:29–35
Wojczulanis-Jakubas K, Jakubas D (2012) When and why does my mother leave me? The question of brood desertion in the Dovekie (Alle alle). Auk 129:632–637
Wojczulanis-Jakubas K, Jakubas D, Øigarden T, Lifjeld JT (2009a) Extra-pair copulations are frequent but unsuccessful in a highly colonial seabird, the little auk, Alle alle. Anim Behav 77:433–438
Wojczulanis-Jakubas K, Jakubas D, Stempniewicz L (2009b) Sex-specific parental care by incubating little auks (Alle alle). Ornis Fenn 86:140–148
Wojczulanis-Jakubas K, Jakubas D, Kidawa D, Kośmicka A (2012) Is the transition from biparental to male-only care in a monogamous seabird related to changes in body mass and stress level? J Ornithol 153:793–800
Wojczulanis-Jakubas K, Jakubas D, Chastel O (2013) Behavioural and hormonal stress responses during chick rearing do not predict brood desertion by female in a small Arctic seabird. Horm Behav 64:448–453
Wojczulanis-Jakubas K, Jakubas D, Chastel O (2014a) Different tactics, one goal: initial reproductive investments of males and females in a small Arctic seabird. Behav Ecol Sociobiol 68:1521–1530
Wojczulanis-Jakubas K, Jakubas D, Kulaszewicz I, Kidawa D, Taylor JRE (2014b) Influence of primary reproductive investments on blood biochemistry, leukocyte profile, and body mass in a small Arctic seabird. Auk 131:743–755
Yu-Lee LY (2002) Prolactin modulation of immune and inflammatory responses. Recent Prog Horm Res 57:435–455
Acknowledgments
The study was supported by a Grant from the Polish Ministry of Science and Higher Education (Iuventus Plus 0470/P01/2010/70 to KWJ). At the CEBC, we thank C. Parenteau and C. Trouvé for their excellent technical assistance in hormone assays. Thanks also go to Peter Senn for linguistic support and three anonymous reviewers for helpful comments that helped to improve the manuscript. All fieldwork has been performed by kind permission of the Norwegian Animal Research Authority and the Governor of Svalbard.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Wojczulanis-Jakubas, K., Jakubas, D., Chastel, O. et al. A big storm in a small body: seasonal changes in body mass, hormone concentrations and leukocyte profile in the little auk (Alle alle). Polar Biol 38, 1203–1212 (2015). https://doi.org/10.1007/s00300-015-1687-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-015-1687-y