Summary
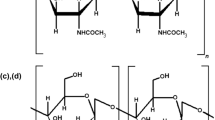
A series of O-(acyl) chitosan (OAC) derivatives with a degree of substitution (DS) between 0.02 and 0.28 were synthesized by reaction of alkanoic acid derivatives with chitosan in the presence of H2SO4 as a catalyst. The reaction was performed at 80 °C for 4 h with different mol ratios of alkanoic acids to chitosan. The synthesized compounds were analyzed by 1H- and 13C-NMR spectroscopy. A high DS was obtained with O-(butyroyl) chitosan (DS 0.28) at a mol ratio of (1:5) chitosan to butyric acid. Their fungicidal activity against the grey mould Botrytis cinerea (Leotiales: Sclerotiniaceae) and the rice leaf blast pathogen Pyricularia grisea (Teleomorph: Magnaportha grisea) has been evaluated. O-(decanoyl) chitosan at mol ratio of 1:2 (chitosan to decanoic acid) was the most active compound against B. cinerea (EC50=1.02 g.l-1) and O-(hexanoyl) chitosan displayed the highest activity against P. grisea (EC50=1.11 g.l-1). It has been mentioned that some derivatives also repressed spore formation at rather high concentrations (1.0, 2.0 and 5.0 g.l-1). The insecticidal activity has been screened at 5 g.kg-1 artificial diet against the larvae of the cotton leafworm Spodoptera littoralis (Lepidoptera: Noctuidae). The results revealed that all of the synthesized derivatives showed high inhibition of growth of the larvae of S. littoralis compared to chitosan (7% growth inhibition) and the most active one was O-(decanoyl) chitosan (64% growth inhibition) after 5 days of feeding on treated artificial diet.
Similar content being viewed by others
References
Francis SJK, Matthew HWT (2000) Biomat 21: 2589
Ravi Kumar NVM (2000) React Funct Polym 46: 1
Sigh DK, Ray AR (2000) Macromol Chem Phys C40: 69
Li Q, Dum ET, Grandmaison EW, Goodman MFA (1992) J Bioact Compat Polym 7: 370
Chandy T, Sharma CP (1990) Biomat Art Cells Art Org 18: 1
Marguerite R, Pham LD, Claude G (1992) Int J Biol Macromol 14: 122
Muzzarelli RAA, Tanfani F (1985) Carbohydr Polym 5: 297
Kas HSJ (1997) Microencapsulation 14: 689
Kubota B, Kikuchi Y In Polysaccharides: Structural Diversity and Functional Versatility; Dumitriu S, Ed Marcel Dekker New York (1998) p 595
Riccardo A, Muzzarelli A (1988) Carbohydr Polym 8: 1
Lillo LE, Matsuhiro B (1997) Carbohydr Polym 34:397
Sashiwa H, Kawasaki N, Nakayama A, Muraki E, Yajima H, Yamamori N, Ichinose Y, Sunamoto J, Aiba S (2003) Carbohydr Res 338: 557
Skorik Y, Gomes CAR, Vasconcelos TSD, Yatluk YG (2003) Carbohydr Res 338: 271
Holme KR, Perlin AS (1997) Carbohydr Res 302: 173
Focher B, Massoli A, Torri G, Gervasini A, Morazzoni F (1986) Macromol Chem 187: 2609
Grant S, Blair H, Mckay G (1988) Polym Commun 29: 342
Heras A, Rodriguez NM, Ramos VM, Agullo E (2001) Carbohydr Polym 44: 1
Ramos VM, Rodriguez NM, Diaz MF, Rodriguez MS, Heras A, Agullo E (2003) Carbohydr Polym 52: 39
Rabea EI, Badawy MEI, Rogge TM, Stevens CV, Smagghe G, Höfte M, Steurbaut W In Proceedings of the 9th International Chitin-Chitosan Conference, Montereal, Québec, Canada 2003, p 103
Wenming X, Peixin X, Qin L (2002) Carbohydr Polym 50: 35
Zhishen J, Dongfen S, Weiliang X (2001) Carbohydr Res 333: 1
Song Y, Onishi H, Nagai T (1992) Chem Pharm Bull 40: 2822
Zhang C, Qineng P, Hongjuan Z, Jian S (2003) Eur Polym J 39: 1629
Rabea EI, Badawy MEI, Stevens CV, Smagghe G, Steurbaut W (2003) Biomacromol 4: 1457
Badawy MEI, Rabea EI, Rogge TM, Stevens CV, Smagghe G, Steurbaut W, Höfte M (2004) Biomacromol 5: 589
Rabea EI, Badawy MEI, Rogge TM, Stevens CV, Smagghe G, Höfte M, Steurbaut W In Proceedings of the 9th International Chitin-Chitosan Conference, Montereal, Québec, Canada 2003, p 104
Rabea EI, Badawy MEI, Rogge TM, Stevens CV, Smagghe G, Hofte M, Steurbaut W (2003) Comm Agric Appl Bio Sci (Ghent University) 68: 135
Rabea EI, Badawy MEI, Rogge TM, Stevens CV, Smagghe G, Höfte M, Steurbaut W (2004) Advances in Chitin Sci 7: 231
Liu XF, Guan YL, Yang DZ, Li Z, Yao K,D (2001) J Appl Polym Sci 79: 1324
Cabrera G, Casals P, Cardenas G, Taboada E, Neira P (2001) In Proc 10th IUPAC Int Congress Chem Crop Protec 1: 227
Cardenas G, Paredes JC, Cabrera G, Casals PJ (2002) Appl Polym Sci 86: 2742
Casals P, Cardenas G, Galvez G, Villar A, Cabrera G (2002) In Proc 10th IUPAC Int Congress Chem Crop Protec 1: 228
Placencia J, Rudolph A, Cabrera G, Cardenas G, Parra C (2003) Bull Environ Contam Toxicol 70: 53
Zhang MI, Tan T, Yuan H, Rui CJ (2003) Bioactive Compatible Polym 18: 391
Stossel P, Leuba JL (1984) J Phytopathol 111: 82
El Ghaouth EA, Arul J, Grenier J, Asselin A (1992) Phytopathol 82: 398
Finney DJ Probit analysis. 3rd Ed. Cambridge University Press Cambridge (1971) p 318
Smagghe G, Carton B, Decombel L, Tirry L (2001) Arch Insect Biochem Physiol 46: 127
Smagghe G, Decombel L, Carton B, Voigt B, Adam G, Tirry L (2002) Insect Biochem Mol Biol 32: 199
Kim JH, Lee YM (1993) Polym 34: 1952
Jin CW, Kim CH, Choi KS (1995) J Korean Ind Eng Chem 6: 482
Sashiwa H, Norioki K, Atsuyoshi N, Einosuke M, Noboru Y, Hong Z, Hiroshi N, Yoshihiko O, Hiroyuki S, Yoshihiro S, Sei-ichi A (2002) Biomacromol 3: 1120
Sashiwa H, Norioki K, Atsuyoshi N, Einosuke M, Noboru Y, Sei-ichi A (2002) Biomacromol 3: 1126
Seo T, Ikeda Y, Torada K, Nakata Y, Shimomura Y (2001) Chitin Chitosan Res 7: 212
Inoue K, Yoshizawa K, Othto K, Nakagawa H (2001) Chem Lett 30: 698
Nishimura S, Kohgo O, Kurita K, Vittavatvong C, Kuwahara K (1990) Chem Lett 19: 243
Du J, Gemma H, Iwahori S (1997) J Jpn Soc Hortic Sci 66: 15
El Ghaouth A, Arul J, Wilson C, Asselin A, Benhamou N (1994) Mol Plant Pathol 44: 417
Oh SK, Cho D, Yu SH (1998) Korean J Plant Pathol 14: 278
Benhamou N (1996) Trends Plant Sci 1: 233
Debieu D, Bach J, Hugon M, Malosse C, Leroux P (2001) Pest Manag Sci 57: 1067
Allan CR, Hadwiger LA (1979) Exp Mycol 3: 285
Hon DNS In: Polysaccharides in Medicinal Application; Dumitri S Ed Marcel Dekker: New York (1996) p 631
Yokoi H, Aratake T, Nishio S, Hirose J, Hayashi S, Takasaki Y (1998) J Ferment Bioeng 85: 246
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Badawy, M., Rabea, E., Rogge, T. et al. Fungicidal and Insecticidal Activity of O-Acyl Chitosan Derivatives. Polym. Bull. 54, 279–289 (2005). https://doi.org/10.1007/s00289-005-0396-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-005-0396-z