Abstract
Purpose
In this study, we characterize the population pharmacokinetics of kahalalide F (KF), a novel marine anticancer drug, after intravenous (i.v.) administration in advanced cancer patients.
Methods
Data from 240 patients included in three Phase I and three Phase II trials receiving i.v. weekly and every 3 weeks infusions of KF, at doses ranging 266–6650 µg/m2, were analyzed using NONMEM™ VII. The effect of demographics and/or pathophysiologically relevant factors on KF pharmacokinetic parameters was evaluated. Model evaluation was conducted using nonparametric bootstrap and visual predictive check (in both internal and external datasets).
Results
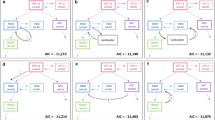
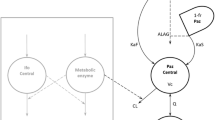
An open two-compartment model with linear distribution and elimination from central compartment was suitable to describe the data. Volume of distribution at steady state and its between-subject variability (CV%) was estimated to be 6.56 L (28 %). Plasma clearance was estimated to be 6.25 L/h (43 %). Within the range of covariates evaluated, age, weight, body surface area, gender, ECOG performance status, presence of liver metastases, creatinine clearance, alkaline phosphatase, aspartate aminotransferase, alanine aminotransferase, total bilirubin, total protein and serum albumin did not contribute to explain KF pharmacokinetic variability to a significant extent. The developed model was deemed appropriate to describe the time course of KF plasma concentrations and its variability in advanced cancer patients.
Conclusion
The integration of pharmacokinetic data from six clinical studies demonstrated KF linear elimination from plasma, dose-proportional exposure and time-independent pharmacokinetics. Based on analyzed data, no clinically relevant covariates were identified as predictors of KF pharmacokinetics.



Similar content being viewed by others
References
Gao J, Hamann MT (2011) Chemistry and biology of kahalalides. Chem Rev 111(5):3208–3235. doi:10.1021/cr100187n
Bonnard I, Manzanares I, Rinehart KL (2003) Stereochemistry of Kahalalide F. J Nat Prod 66(11):1466–1470. doi:10.1021/np030334c
Gracia C, Isidro-Llobet A, Cruz LJ, Acosta GA, Alvarez M, Cuevas C, Giralt E, Albericio F (2006) Convergent approaches for the synthesis of the antitumoral peptide, Kahalalide F. Study of orthogonal protecting groups. J Org Chem 71(19):7196–7204. doi:10.1021/jo060976f
Hamann MT (2004) Technology evaluation: Kahalalide F, PharmaMar. Curr Opin Mol Ther 6(6):657–665
Hamann MT, Otto CS, Scheuer PJ, Dunbar DC (1996) Kahalalides: Bioactive Peptides from a Marine Mollusk Elysia rufescens and its Algal Diet Bryopsis sp. (1). J Org Chem 61(19):6594–6600
Lopez-Macia A, Jimenez JC, Royo M, Giralt E, Albericio F (2001) Synthesis and structure determination of Kahalalide F (1,2). J Am Chem Soc 123(46):11398–11401
Serova M, de Gramont A, Bieche I, Riveiro ME, Galmarini CM, Aracil M, Jimeno J, Faivre S, Raymond E (2013) Predictive factors of sensitivity to elisidepsin, a novel Kahalalide F-derived marine compound. Mar Drugs 11(3):944–959. doi:10.3390/md11030944
Salazar R, Jones RJ, Oaknin A, Crawford D, Cuadra C, Hopkins C, Gil M, Coronado C, Soto-Matos A, Cullell-Young M, Iglesias Dios JL, Evans TR (2012) A Phase I and pharmacokinetic study of elisidepsin (PM02734) in patients with advanced solid tumors. Cancer Chemother Pharmacol 70(5):673–681. doi:10.1007/s00280-012-1951-6
Salazar R, Cuadra C, Gil-Martin M, Vandermeeren A, Alfaro V, Coronado C (2012) Complete and sustained objective response per RECIST to Irvalec (PM02734) in undifferentiated large cell esophageal adenocarcinoma: a case report and a review of the literature. Case Rep Oncol 5(2):354–358. doi:10.1159/000341104
Provencio M, Sanchez A, Gasent J, Gomez P, Rosell R (2009) Cancer treatments: Can we find treasures at the bottom of the sea? Clin Lung Cancer 10(4):295–300. doi:10.3816/CLC.2009.n.041
Garcia-Rocha M, Bonay P, Avila J (1996) The antitumoral compound Kahalalide F acts on cell lysosomes. Cancer Lett 99(1):43–50
Sewell JM, Mayer I, Langdon SP, Smyth JF, Jodrell DI, Guichard SM (2005) The mechanism of action of Kahalalide F: variable cell permeability in human hepatoma cell lines. Eur J Cancer 41(11):1637–1644. doi:10.1016/j.ejca.2005.04.015
Shilabin AG, Hamann MT (2011) In vitro and in vivo evaluation of select Kahalalide F analogs with antitumor and antifungal activities. Bioorganic Med Chem 19(22):6628–6632. doi:10.1016/j.bmc.2011.06.050
Cruz LJ, Luque-Ortega JR, Rivas L, Albericio F (2009) Kahalalide F, an antitumor depsipeptide in clinical trials, and its analogues as effective antileishmanial agents. Mol Pharm 6(3):813–824. doi:10.1021/mp8001039
Suarez Y, Gonzalez L, Cuadrado A, Berciano M, Lafarga M, Munoz A (2003) Kahalalide F, a new marine-derived compound, induces oncosis in human prostate and breast cancer cells. Mol Cancer Ther 2(9):863–872
Brown AP, Morrissey RL, Faircloth GT, Levine BS (2002) Preclinical toxicity studies of kahalalide F, a new anticancer agent: single and multiple dosing regimens in the rat. Cancer Chemother Pharmacol. 50(4):333–340
Martin-Algarra S, Espinosa E, Rubio J, Lopez Lopez JJ, Manzano JL, Carrion LA, Plazaola A, Tanovic A, Paz-Ares L (2009) Phase II study of weekly Kahalalide F in patients with advanced malignant melanoma. Eur J Cancer 45(5):732–735. doi:10.1016/j.ejca.2008.12.005
Pardo B, Paz-Ares L, Tabernero J, Ciruelos E, Garcia M, Salazar R, Lopez A, Blanco M, Nieto A, Jimeno J, Izquierdo MA, Trigo JM (2008) Phase I clinical and pharmacokinetic study of Kahalalide F administered weekly as a 1-hour infusion to patients with advanced solid tumors. Clin Cancer Res Off J Am Assoc Cancer Res 14(4):1116–1123. doi:10.1158/1078-0432.CCR-07-4366
Rademaker-Lakhai JM, Horenblas S, Meinhardt W, Stokvis E, de Reijke TM, Jimeno JM, Lopez-Lazaro L, Lopez Martin JA, Beijnen JH, Schellens JH (2005) Phase I clinical and pharmacokinetic study of Kahalalide F in patients with advanced androgen refractory prostate cancer. Clin Cancer Res Off J Am Assoc Cancer Res 11(5):1854–1862. doi:10.1158/1078-0432.CCR-04-1534
Salazar R, Cortes-Funes H, Casado E, Pardo B, Lopez-Martin A, Cuadra C, Tabernero J, Coronado C, Garcia M, Soto Matos-Pita A, Miguel-Lillo B, Cullell-Young M, Iglesias Dios JL, Paz-Ares L (2013) Phase I study of weekly Kahalalide F as prolonged infusion in patients with advanced solid tumors. Cancer Chemother Pharmacol 72(1):75–83. doi:10.1007/s00280-013-2170-5
Sparidans RW, Stokvis E, Jimeno JM, Lopez-Lazaro L, Schellens JH, Beijnen JH (2001) Chemical and enzymatic stability of a cyclic depsipeptide, the novel, marine-derived, anti-cancer agent Kahalalide F. Anticancer Drugs 12(7):575–582
Piggott AM, Karuso P (2008) Rapid identification of a protein binding partner for the marine natural product Kahalalide F by using reverse chemical proteomics. Chembiochem Eur J Chem Biol 9(4):524–530. doi:10.1002/cbic.200700608
Stokvis E, Rosing H, Lopez-Lazaro L, Rodriguez I, Jimeno JM, Supko JG, Schellens JH, Beijnen JH (2002) Quantitative analysis of the novel depsipeptide anticancer drug Kahalalide F in human plasma by high-performance liquid chromatography under basic conditions coupled to electrospray ionization tandem mass spectrometry. J Mass Spectrom JMS 37(9):992–1000. doi:10.1002/jms.362
Stokvis E, Rosing H, Lopez-Lazaro L, Schellens JH, Beijnen JH (2004) Switching from an analogous to a stable isotopically labeled internal standard for the LC–MS/MS quantitation of the novel anticancer drug Kahalalide F significantly improves assay performance. Biomed Chromatogr BMC 18(6):400–402. doi:10.1002/bmc.392
Beal S, Sheiner LB, Boeckmann A, Bauer RJ (2009) NONMEM user’s guides (1989–2009). Icon Development Solutions, Ellicott City, MD
Keizer RJ, Karlsson MO, Hooker A (2013) Modeling and simulation workbench for NONMEM: tutorial on Pirana, PsN, and Xpose. CPT Pharmacomet Syst Pharmacol 2:e50. doi:10.1038/psp.2013.24
Lindbom L, Ribbing J, Jonsson EN (2004) Perl-speaks-NONMEM (PsN)—a Perl module for NONMEM related programming. Comput Methods Programs Biomed 75(2):85–94
R Development Core Team (2011) R: a language and environment for statistical computing. The R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/
Ahn JE, Karlsson MO, Dunne A, Ludden TM (2008) Likelihood based approaches to handling data below the quantification limit using NONMEM VI. J Pharmacokinet Pharmacodyn 35(4):401–421. doi:10.1007/s10928-008-9094-4
Beal SL (2001) Ways to fit a PK model with some data below the quantification limit. J Pharmacokinet Pharmacodyn 28(5):481–504
Bergstrand M, Karlsson MO (2009) Handling data below the limit of quantification in mixed effect models. AAPS J 11(2):371–380. doi:10.1208/s12248-009-9112-5
Karlsson MO, Savic RM (2007) Diagnosing model diagnostics. Clin Pharmacol Ther 82(1):17–20. doi:10.1038/sj.clpt.6100241
Cockcroft DW, Gault MH (1976) Prediction of creatinine clearance from serum creatinine. Nephron 16(1):31–41
Alfaro ME, Zoller S, Lutzoni F (2003) Bayes or bootstrap? A simulation study comparing the performance of Bayesian Markov chain Monte Carlo sampling and bootstrapping in assessing phylogenetic confidence. Mol Biol Evol 20(2):255–266
Efron B (2011) The bootstrap and Markov-chain Monte Carlo. J Biopharm Stat 21(6):1052–1062. doi:10.1080/10543406.2011.607736
Yano Y, Beal SL, Sheiner LB (2001) Evaluating pharmacokinetic/pharmacodynamic models using the posterior predictive check. J Pharmacokinet Pharmacodyn 28(2):171–192
Brendel K, Comets E, Laffont C, Laveille C, Mentre F (2006) Metrics for external model evaluation with an application to the population pharmacokinetics of gliclazide. Pharm Res 23(9):2036–2049. doi:10.1007/s11095-006-9067-5
Perez-Ruixo JJ, Zannikos P, Hirankarn S, Stuyckens K, Ludwig EA, Soto-Matos A, Lopez-Lazaro L, Owen JS (2007) Population pharmacokinetic meta-analysis of trabectedin (ET-743, Yondelis) in cancer patients. Clin Pharmacokinet 46(10):867–884
Nalda-Molina R, Valenzuela B, Ramon-Lopez A, Miguel-Lillo B, Soto-Matos A, Perez-Ruixo JJ (2009) Population pharmacokinetics meta-analysis of plitidepsin (Aplidin) in cancer subjects. Cancer Chemother Pharmacol 64(1):97–108. doi:10.1007/s00280-008-0841-4
van Hasselt JG, Gupta A, Hussein Z, Beijnen JH, Schellens JH, Huitema AD (2013) Population pharmacokinetic-pharmacodynamic analysis for eribulin mesilate-associated neutropenia. Br J Clin Pharmacol 76(3):412–424. doi:10.1111/bcp.12143
Perez-Ruixo C, Valenzuela B, Fernandez Teruel C, Gonzalez-Sales M, Miguel-Lillo B, Soto-Matos A, Perez-Ruixo JJ (2012) Population pharmacokinetics of PM00104 (Zalypsis((R))) in cancer patients. Cancer Chemother Pharmacol 69(1):15–24. doi:10.1007/s00280-011-1644-6
Gabrielsson J, Weiner D (2012) Non-compartmental analysis. In: Reisfeld B, Mayeno AN (eds) Computational toxicology, methods in molecular biology, vol 929. Humana Press, New York City, pp 377–389. doi:10.1007/978-1-62703-050-2_16
Savic RM, Karlsson MO (2009) Importance of shrinkage in empirical bayes estimates for diagnostics: problems and solutions. AAPS J 11(3):558–569. doi:10.1208/s12248-009-9133-0
Mathijssen RH, de Jong FA, Loos WJ, van der Bol JM, Verweij J, Sparreboom A (2007) Flat-fixed dosing versus body surface area based dosing of anticancer drugs in adults: Does it make a difference? Oncologist 12(8):913–923. doi:10.1634/theoncologist.12-8-913
Acknowledgments
The authors would like to thank the hundreds of patients, investigators and their medical, nursing and laboratory staff that participated in the clinical studies included in the present pooled study. We also thank Hilde Rosing and Josh H. Beijnen (Slootervart Hospital, Amsterdam, the Netherlands) for their bioanalysis work provided during this development of KF.
Conflict of interest
Bernardo Miguel-Lillo and Arturo Soto-Matos are employees of Pharma Mar SA, which supported this study. No other conflict of interest was declared.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Miguel-Lillo, B., Valenzuela, B., Peris-Ribera, J.E. et al. Population pharmacokinetics of kahalalide F in advanced cancer patients. Cancer Chemother Pharmacol 76, 365–374 (2015). https://doi.org/10.1007/s00280-015-2800-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-015-2800-1