Abstract
Purpose
S-1 has a favorable effect in unresectable pancreatic cancer and a potential radiosensitizer. In addition, daily oral administration of S-1 is more convenient than continuous infusion of 5-fluorouracil. This study was designed to evaluate the efficacy and safety of S-1 and concurrent radiotherapy in patients with locally advanced pancreatic cancer.
Methods
Eligibility criteria were histologically proven pancreatic adenocarcinoma, locally advanced disease, and no previous treatment. S-1 was administered orally at a dose of 40 mg/m2 twice daily from day 1 to 14 and from day 22 to 35, and concurrent radiotherapy (a total dose of 50.4 Gy) was delivered in 28 fractions. One month after treatment completion, tumor response was evaluated by computed tomography (CT).
Results
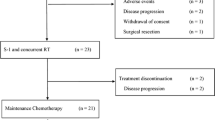
A total of 25 patients were evaluable for efficacy and toxicity on the basis of the intention-to-treat analysis. The response rate and disease control rate were 24.0 and 68.0%, respectively. There was no treatment-related death or grade 4 toxicity. The most common grade 3 hematologic and non-hematologic toxicities were thrombocytopenia (4.0%) and anorexia (20%), respectively. All toxicities were tolerable and transient. The median time-to-progression and median overall survival were 6.5 months (95% confidence interval, 4.1–9.0 months) and 12.9 months (95% confidence interval, 6.7–19.0 months), respectively, and the 1-year survival rate was estimated to be 43%.
Conclusions
S-1 and concurrent radiotherapy shows favorable efficacy for disease control against locally advanced pancreatic cancer and was well tolerated with no severe toxicities.

Similar content being viewed by others
References
A’Hern RP (2001) Sample size table for exact single-stage phase II designs. Stat Med 20:859–866
Bang S, Chung HW, Park SW, Chung JB, Yun M, Lee JD, Song SY (2006) The clinical usefulness of 18-fluorodeoxyglucose positron emission tomography in the differential diagnosis, staging, and response evaluation after concurrent chemoradiotherapy for pancreatic cancer. J Clin Gastroenterol 40:923–929
Burris HA, Moore MJ, Anderson J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, Von Hoff DD (1997) Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 15:2403–2413
Choi HJ, Kim NK, Keum KC, Cheon SH, Shin SJ, Baik SH, Choen JH, Rha SY, Roh JK, Jeung HC, Chung HC, Ahn JB (2008) Phase I trial of neoadjuvant concurrent chemoradiotherapy with S-1 and weekly irinotecan in locally advanced rectal cancer. Radiother Oncol 87:361–366
Crane CH, Varadhachary G, Pisters PWT, Evans DB, Wolff RA (2007) Future chemoradiation strategies in pancreatic cancer. Semin Oncol 34:335–346
Gastrointestinal Tumor Study Group (1988) Treatment of locally unresectable carcinoma of the pancreas: comparison of combined-modality therapy (chemotherapy plus radioatherapy) to chemotherapy alone. J Natl Cancer Inst 80:751–755
Harada K, Ferdous T, Yoshida H (2007) Investigation of optimal schedule of concurrent radiotherapy with S-1 for oral squamous cell carcinoma. Oncol Rep 18:1077–1083
Harada K, Kawaguchi S, Supriatno , Kawashima Y, Yoshida H, Sato M (2005) S-1, an oral fluoropyrimidine anti-cancer agent, enhanced radiosensitivity in a human oral cancer cell line in vivo and in vitro: involvement possibility of inhibition of survival signal, Akt/PKB. Cancer Lett 226:161–168
Harada K, Kawaguchi S, Supriatno , Onoue T, Yoshida H, Sato M (2004) Combined effects of the oral fluoropyrimidine anticancer agent, S-1 and radiation on human oral cancer cells. Oral Oncol 40:713–719
Hirata K, Horikoshi N, Aiba K, Okazaki M, Denno R, Sasaki K, Nakano Y, Ishizuka H, Yamada Y, Uno S, Taguchi T, Shirasaka T (1995) Pharmacokinetic study of S-1, a novel oral fluorouracil antitumor drug. Clin Cancer Res 5:2000–2005
Huguet F, André T, Hammel P, Artru P, Balosso J, Selle F, Deniaud-Alexandre E, Ruszniewski P, Touboul E, Labianca R, de Gramont A, Louvet C (2007) Impact of chemoradiotherapy after disease control with chemotherapy in locally advanced pancreatic adenocarcinoma in GERCOR phase II and III studies. J Clin Oncol 25:326–331
Ikeda M, Okusaka T, Ito Y, Ueno H, Morizane C, Furuse F, Ishii H, Kawashima M, Kagami Y, Ikeda H (2007) A phase I trial of S-1 with concurrent radiotherapy for locally advanced pancreatic cancer. Br J Cancer 96:1650–1655
Ishii H, Furuse J, Nakachi K, Suzuki E, Yoshino M (2005) Primary tumor of pancreatic cancer as a measurable target lesion in chemotherapy trials. Jpn J Clin Oncol 35:601–606
Kim R, Saif MW (2007) Is there an optimal neoadjuvant therapy for locally advanced pancreatic cancer? J Pancreas 8:279–288
Kimura Y, Kikkawa N, Iijima S, Kato T, Naoi Y, Hayashi T, Tanigawa T, Yamamoto H, Kurokawa E (2003) A new regimen for S-1 therapy aiming at adverse reaction mitigation and prolonged medication by introducing a 1-week drug-free interval after each 2-week dosing session: efficacy and feasibility in clinical practice. Gastric Cancer 6(Suppl 1):34–39
Klaassen DJ, MacIntyre JM, Catton GE, Engstrom PF, Moertel CG (1985) Treatment of locally unresectable cancer of the stomach and pancreas: a randomized comparison of 5-fluorouracil alone with radiation plus concurrent and maintenance 5-fluorouracil—an Eastern Cooperative Oncology Group study. J Clin Oncol 3:373–378
Lawrence TS, Chang EY, Hahn TM, Hertel LW, Shewach DS (1996) Radiosensitization of pancreatic cancer cells by 2′,2′-difluoro-2′-deoxycytidine. Int J Radiat Oncol Biol Phys 34:867–872
Moertel CG, Childs DS Jr, Reitemeier RJ, Colby MY Jr, Holbrook MA (1969) Combined 5-fluorouracil and supervoltage radiation therapy of locally unresectable gastrointestinal cancer. Lancet 2:865–867
Moertel CG, Frytak S, Hahn RG, O’Connell MJ, Reitemeier RJ, Rubin J, Schutt AJ, Weiland LH, Childs DS, Holbrook MA, Lavin PT, Livstone E, Spiro H, Knowlton A, Kalser M, Barkin J, Lessner H, Mann-Kaplan R, Ramming K, Douglas HO Jr, Thomas P, Nave H, Bateman J, Lokich J, Brooks J, Chaffey J, Corson JM, Zamcheck N, Novak JW (1981) Therapy of locally unresectable pancreatic carcinoma: a randomized comparison of high dose (6000 rads) radiation alone, moderate dose radiation (4,000 rads + 5-fluorouracil), and high dose radiation + 5-fluorouracil: The Gastrointestinal Tumor Study Group. Cancer 48:1705–1710
Moureau-Zabotto L, Phélip JM, Afchain P, Mineur L, André T, Vendrely V, Lledo G, Dupuis O, Huguet F, Touboul E, Balosso J, Louvet C (2008) Concomitant administration of weekly oxaliplatin, fluorouracil continuous infusion, and radiotherapy after 2 months of gemcitabine and oxaliplatin induction in patients with locally advanced pancreatic cancer: a Groupe Coordinateur Multidisciplinaire en Oncologie phase II study. J Clin Oncol 26:1080–1085
Nakata E, Fukushima M, Takai Y, Nemoto K, Ogawa Y, Nomiya T, Nakamura Y, Milas L, Yamada S (2006) S-1, an oral fluoropyrimidine, enhances radiation response of DLD-1/FU human colon cancer xenografts resistant to 5-FU. Oncol Rep 16:465–471
Ohtsu A, Baba H, Sakata Y, Mitachi Y, Horikoshi N, Sugimachi K, Taguchi T (2000) Phase II study of S-1, a novel oral fluoropyrimidine derivative, in patients with metastatic colorectal carcinoma. Br J Cancer 83:141–145
Okusaka T, Funakoshi A, Furuse J, Boku N, Yamao K, Ohkawa S, Saito H (2008) A late phase II study of S-1 for metastatic pancreatic cancer. Cancer Chemother Pharmacol 61:615–621
Oya N (2004) Chemoradiotherapy for pancreatic cancer: current status and perspectives. Int J Clin Oncol 9:451–457
Sultana A, Tudur Smith C, Cunningham D, Starling N, Neoptolemos JP, Ghaneh P (2008) Meta-analyses of chemotherapy for locally advanced and metastatic pancreatic cancer: results of secondary end points analyses. Br J Cancer 99:6–13
Sakata Y, Ohtsu A, Horikoshi N, Sugimachi K, Mitachi Y, Taguchi T (1998) Late phase II study of novel oral fluoropyrimidine anticancer drug S-1 (1 M tegafur-0.4 M gimestat-1 M otastat potassium) in advanced gastric cancer patients. Eur J Cancer 34:1715–1720
Shinchi H, Maemura K, Noma H, Mataki Y, Aikou T, Takao S (2007) Phase-I trial of oral fluoropyridine anticancer agent (S-1) with concurrent radiotherapy in patients with unresectable pancreatic cancer. Br J Cancer 96:1353–1357
Shirasaka T, Shimamato Y, Ohshimo H, Yamaguchi M, Kato T, Yonekura K, Fukushima M (1996) Development of a novel form of an oral 5-fluorouracil derivative (S-1) directed to the potentiation of the tumour selective cytotocitiy of 5-fluorouracil by two biochemical modulators. Anticancer Drugs 7:548–557
Sudo K, Yamaguchi T, Ishihara T, Nakamura K, Shirai Y, Akihiko N, Kawakami H, Uno T, Ito H, Saisho H (2007) Phase I study of oral S-1 and concurrent radiotherapy in patients with unresectable locally advanced pancreatic cancer. Int J Radiation Oncology Biol Phys 67:219–224
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Tsuji H, Kiba T, Nagata M, Inoue T, Yukawa H, Yamashita T, Shimode Y, Murata H, Nagata K, Tomoda K (2006) A phase I study of concurrent chemoradiotherapy with S-1 for T2N0 glottic carcinoma. Oncology 71:369–373
Ueno H, Okusaka T, Ikeda M, Takezako Y (2005) An early phase II study of S-1 in patients with metastatic pancreatic cancer. Oncology 68:171–178
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, H.M., Bang, S., Park, J.Y. et al. Phase II trial of S-1 and concurrent radiotherapy in patients with locally advanced pancreatic cancer. Cancer Chemother Pharmacol 63, 535–541 (2009). https://doi.org/10.1007/s00280-008-0836-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-008-0836-1