Abstract
Purpose
To determine whether primary stenting reduces the rate of restenosis compared with balloon angioplasty alone in the endovascular treatment of long superficial femoral artery lesions; and to assess the effect of treatment on quality of life.
Methods
A total of 150 patients with superior femoral artery occlusion or severe stenosis of 5–22 cm length from 17 UK centers were randomized to either primary stenting with the SMART stent or balloon angioplasty (i.e., percutaneous transluminal angioplasty, PTA). Bailout stent placement was permitted in case of inadequate result from PTA. The primary end point was restenosis measured by duplex ultrasound at 1 year. Quality-of-life assessments were performed by the EuroQol (EQ)-5D questionnaire.
Results
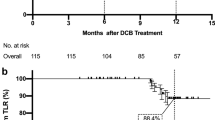
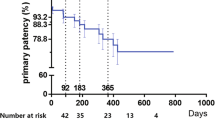
Mean lesion length was 123.0 mm in the stent group and 116.8 mm in the PTA group. A total of 140 (93.3 %) of 150 had total occlusions. At 12 months’ follow-up, restenosis measured by Duplex ultrasound was not significantly different between the stent and PTA groups by intention-to-treat or as-treated analyses: 47.2 versus 43.5 % (p = 0.84) and 40.8 versus 46.7 % (p = 0.68), respectively. There were fewer target lesion revascularizations in patients randomized to stenting, but this did not reach statistical significance (12.5 vs. 20.8 %, p = 0.26). There was no difference in the rate of amputation. Patients in both groups reported improved quality of life.
Conclusion
Primary stenting of long lesions in predominantly occluded superficial femoral arteries does not reduce the rate of binary restenosis compared with balloon angioplasty and bailout stenting. Both treatment strategies conferred a meaningful and sustained improvement to the quality of life of patients with severe superficial femoral artery disease.


Similar content being viewed by others
References
Duda SH, Pusich B, Richter G et al (2002) Sirolimus-eluting stents for the treatment of obstructive superficial femoral artery disease: six-month results. Circulation 106:1505–1509
Duda SH, Bosiers M, Lammer J et al (2005) Sirolimus-eluting versus bare nitinol stent for obstructive superficial femoral artery disease: the SIROCCO II trial. J Vasc Interv Radiol 16:331–338
Mewissen MW (2004) Self-expanding Nitinol stents in the femoropopliteal segment: technique and mid-term results. Tech Vasc Interv Radiol 7:2–5
Vogel TR, Shindelman LE, Nackman GB, Graham AM (2003) Efficacious use of nitinol stents in the femoral and popliteal arteries. J Vasc Surg 38:1178–1184
Schillinger M, Sabeti S, Loewe C et al (2006) Balloon angioplasty versus implantation of nitinol stents in the superficial femoral artery. N Engl J Med 354:1879–1888
EuroQol Group (1990) EuroQol—a new facility for the measurement of health-related quality of life. Health Policy 16:199–208
Dolan P, Gudex C, Kind P, Williams A (1995) A social tariff for EuroQol: results from a UK general population survey. Centre for Health Economics Discussion Paper 138. CHE, Centre for Health Economics, University of York
Krankenberg H, Schlüter M, Steinkamp HJ et al (2007) Nitinol stent implantation versus percutaneous transluminal angioplasty in superficial femoral artery lesions up to 10 cm in length: the Femoral Artery Stenting trial (FAST). Circulation 116:285–293
Dick P, Wallner H, Sabeti S et al (2009) Balloon angioplasty versus stenting with nitinol stents in intermediate length superficial femoral artery lesions. Catheter Cardiovasc Interv 74:1090–1095
Laird JR, Katzen BT, Scheinert D et al (2010) Nitinol stent implantation versus balloon angioplasty for lesions in the superficial femoral artery and proximal popliteal artery. Twelve-month results from the RESILIENT randomized trial. Circ Cardiovasc Interv 3:267–276
Twine CP, Coulston J, Shandall A et al (2009) Angioplasty versus stenting for superficial femoral artery lesions. Cochrane Database Syst Rev 15(2):CD006767
Sabeti S, Schillinger M, Amighi J et al (2004) Primary patency of femoropopliteal arteries treated with nitinol versus stainless steel self-expanding stents: propensity score–adjusted analysis. Radiology 232:516–521
Tasc II (2007) Inter-Society Consensus for the management of peripheral arterial disease. Eur J Vasc Endovasc Surg 33:1–75
Davies MG, Bismuth J, We Saad et al (2010) Outcomes of interventions for recurrent disease after endoluminal intervention for superficial femoral artery disease. J Vasc Surg 52:331–339
Cheng SWK, Ting ACW, Wong J (2001) Endovascular stenting of superficial femoral artery stenosis and occlusions: results and risk factor analysis. Cardiovasc Surg 9:133–140
Gray BH, Sullivan TM, Childs MB et al (1997) High incidence of restenosis/reocclusion of stents in the percutaneous treatment of long-segment superficial femoral artery disease after suboptimal angioplasty. J Vasc Surg 25:74–83
Kim SJ, Kim W, Kim JB et al (2010) Factors of success and patency after subintimal angioplasty in patients with TransAtlantic inter-society consensus C and D severe lower extremity occlusive disease. J Am Coll Cardiol 105(9 Suppl 1):20A–21A
Hameed A, Gluege S, Reinhart R (2009) Subintimal angioplasty: to stent or not to stent. J Invasive Cardiol 21:E154–E156
Bosiers M, Deloose K, Callaert J et al (2012) In lower extremity PTAs intraluminal is better than subintimal. J Cardiovasc Surg (Torino) 53:223–227
Schmieder GC, Richardson AI, Scott EC et al (2008) Selective stenting in subintimal angioplasty: analysis of primary stent outcomes. J Vasc Surg 48:1175–1180
Sabeti S, Czerwenka-Wenkstetten A, Dick P et al (2007) Quality of life after balloon angioplasty versus stent implantation in the superficial femoral artery: findings from a randomized controlled trial. J Endovasc Ther 14:431–437
UK Department of Health (2008) Guidance on the routine collection of patient reported outcome measures. Department of Health, London
National Institute for Health and Clinical Excellence (2008) Guide to the methods of technology appraisal. National Institute for Health and Clinical Excellence, London, pp 38–39
de Vries M, Ouwendijk R, Kessels AG et al (2005) Comparison of generic and disease-specific questionnaires for the assessment of quality of life in patients with peripheral arterial disease. J Vasc Surg 41:261–268
BASIL Trial Participants (2005) Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL): multicentre, randomised controlled trial. Lancet 366:1925–1934
Longworth L, Buxton MH, Sculpher M et al (2005) Estimating utility data from clinical indicators for patients with stable angina. Eur J Health Econ 6:347–353
Serruys PW, Unger F, Sousa JE (2001) Comparison of coronary artery bypass surgery and stenting for the treatment of multi-vessel disease. N Engl J Med 344:1117–1124
Dake MD, Ansel GM, Jaff MR et al (2011) Paclitaxel-eluting stents show superiority to balloon angioplasty and bare metal stents in femoropopliteal disease. Circ Cardiovasc Interv 4:495–504
Schlager O, Francesconi M, Haumer M et al (2007) Duplex sonography versus angiography for assessment of femoropopliteal arterial disease in a “real-world” setting. J Endovasc Ther 14:452–459
Schillinger M, Sabeti S, Dick P et al (2007) Sustained benefit at 2 years of primary femoropopliteal stenting compared with balloon angioplasty with optional stenting. Circulation 115:2745–2749
Laird JR, Katzen BT, Scheinert D et al (2012) Nitinol stent implantation versus balloon angioplasty for lesions in the superficial femoral and proximal popliteal arteries of patients with claudication: three-year follow-up from the RESILIENT randomised trial. J Endovasc Ther 19:1–9
Tendera M, Aboyans V, Bartelink ML et al (2011) ESC guidelines on the diagnosis and treatment of peripheral artery diseases. Eur Heart J 32:2851–2906
Acknowledgments
The SUPER study was sponsored and funded by Cordis Clinical Research.
Conflict of interest
SF and MvR are paid employees of Cordis, Johnson & Johnson. The other authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was conducted on behalf of the SUPER study investigators.
List of lead investigators, study site and number of patients recruited.
Appendix: The SUPER study investigators
Appendix: The SUPER study investigators
The Investigators of the above study was conducted on behalf of The SUPER study: N. Chalmers, Manchester Royal Infirmary (n = 33 patients recruited); P. Walker, James Cook University Hospital, Middlesbrough (18); A. Belli, St. George’s Hospital, London (14); A. Thorpe, Aberdeen Royal Infirmary (14); P. Sidhu, King’s College Hospital, London (12); G. Robinson, Hull Royal Infirmary (11); T. Cleveland, Northern General Hospital, Sheffield (9); K. Gill, Pinderfields Hospital, Wakefield (9), R. Ashleigh, Wythenshawe Hospital, Manchester (6); M. Matson, Royal London Hospital (5); J. Cockburn, Norfolk and Norwich University Hospital, Norwich (4); A. Collins, Royal Victoria Hospital, Belfast (4); G. Houston, Ninewells Hospital, Dundee (3); J. Patel, St. James’ Hospital, Leeds (3); M. Cowling, City General Hospital, Stoke-on-Trent (2); J. Moss, Gartnavel Hospital, Glasgow (2); S. Travis, Royal Cornwall Hospital, Truro (1).
Rights and permissions
About this article
Cite this article
Chalmers, N., Walker, P.T., Belli, AM. et al. Randomized Trial of the SMART Stent versus Balloon Angioplasty in Long Superficial Femoral Artery Lesions: The SUPER Study. Cardiovasc Intervent Radiol 36, 353–361 (2013). https://doi.org/10.1007/s00270-012-0492-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-012-0492-z