Abstract
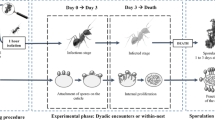
Social groups are at particular risk for parasite infection, which is heightened in eusocial insects by the low genetic diversity of individuals within a colony. To combat this, adult ants have evolved a suite of defenses to protect each other, including the production of antimicrobial secretions. However, it is the brood in a colony that are most vulnerable to parasites because their individual defenses are limited, and the nest material in which ants live is also likely to be prone to colonization by potential parasites. Here, we investigate in two ant species whether adult workers use their antimicrobial secretions not only to protect each other but also to sanitize the vulnerable brood and nest material. We find that, in both leaf-cutting ants and weaver ants, the survival of the brood was reduced and the sporulation of parasitic fungi from them increased, when the workers nursing them lacked functional antimicrobial-producing glands. This was the case for both larvae that were experimentally treated with a fungal parasite (Metarhizium) and control larvae which developed infections of an opportunistic fungal parasite (Aspergillus). Similarly, fungi were more likely to grow on the nest material of both ant species if the glands of attending workers were blocked. The results show that the defense of brood and sanitization of nest material are important functions of the antimicrobial secretions of adult ants and that ubiquitous, opportunistic fungi may be a more important driver of the evolution of these defenses than rarer, specialist parasites.




Similar content being viewed by others
References
Alexander RD (1974) The evolution of social behavior. Annu Rev Ecol Syst 5:325–383
Andersen SB, Ferrari M, Evans HC, Elliot SL, Boomsma JJ, Hughes DP (2012) Disease dynamics in a specialized parasite of ant societies. PLoS One 7:e36352
Armitage SAO, Boomsma JJ (2010) The effects of age and social interactions on innate immunity in a leaf-cutting ant. J Insect Physiol 56:780–787
Baracchi D, Turillazzi S (2010) Differences in venom and cuticular peptides in individuals of Apis mellifera (Hymenoptera: Apidae) determined by MALDI-TOF MS. J Insect Physiol 56:366–375
Baracchi D, Francese S, Turillazzi S (2011) Beyond the antipredatory defence: honey bee venom function as a component of social immunity. Toxicon 58:550–557
Baracchi D, Mazza G, Turillazzi S (2012) From individual to collective immunity: the role of the venom as antimicrobial agent in the Stenogastrinae wasp societies. J Insect Physiol 58:188–193
Blum MS (1992) Ant venoms: chemical and pharmacological properties. Toxin Rev 11:115–164
Boomsma JJ, Schmid-Hempel P, Hughes WOH (2005) Life histories and parasite pressure across the major groups of social insects. In: Holloway G, Rolff J, Fellowes M (eds) Insect Evolutionary Ecology. CABI Publishing, Wallingford, pp 139–176
Bot ANM, Obermayer ML, Hölldobler B, Boomsma JJ (2001) Functional morphology of the metapleural gland in the leaf-cutting ant Acromyrmex octospinosus. Insectes Soc 48:63–66
Bulet P, Hetru C, Dimarcq J-L, Hoffmann D (1999) Antimicrobial peptides in insects; structure and function. Dev Comp Immunol 23:329–344
Chapuisat M, Oppliger A, Magliano P, Christe P (2007) Wood ants use resin to protect themselves against pathogens. Proc R Soc Lond B 274:2013–2017
Cremer S, Armitage SAO, Schmid-Hempel P (2007) Social immunity. Curr Biol 17:693–702
Currie CR (2001) A community of ants, fungi, and bacteria: a multilateral approach to studying symbiosis. Annu Rev Microbiol 55:357–380
Currie CR, Stuart AE (2001) Weeding and grooming of pathogens in agriculture by ants. Proc R Soc Lond B 268:1033–1039
Currie CR, Mueller UG, Malloch D (1999) The agricultural pathology of ant fungus gardens. Proc Natl Acad Sci U S A 96:7998–8002
de Souza AL, Soares IMF, Cyrino LT, Eduardo Serro J (2006) The metapleural gland in two subspecies of Acromyrmex subterraneus (Hymenoptera: Formicidae). Sociobiology 47:19–25
Dornhaus A, Powell S, Bengston S (2010) Group size and its effects on collective organization. Annu Rev Entomol 57:123–141
Ebert D, Zschokke-Rohringer CD, Carius HJ (2000) Dose effects and density-dependent regulation of two microparasites of Daphnia magna. Oecologia 122:200–209
Evans JD, Aronstein K, Chen YP, Hetru C, Imler JL, Jiang H, Kanost M, Thompson GJ, Zou Z, Hultmark D (2006) Immune pathways and defence mechanisms in honey bees Apis mellifera. Insect Mol Biol 15:645–656
Evans HC, Elliot SL, Hughes DP (2011) Hidden diversity behind the zombie-ant fungus Ophiocordyceps unilateralis: four new species described from carpenter ants in Minas Gerais, Brazil. PLoS One 6:e17024
Fernández-Marín H, Zimmerman JK, Rehner SA, Wcislo WT (2006) Active use of the metapleural glands by ants in controlling fungal infection. Proc R Soc Lond B 273:1689–1695
Fernández-Marín H, Zimmerman JK, Nash DR, Boomsma JJ, Wcislo WT (2009) Reduced biological control and enhanced chemical pest management in the evolution of fungus farming in ants. Proc R Soc Lond B 276:2263–2269
Foley K, Fazio G, Jensen AB, Hughes WOH (2012) Nutritional limitation and resistance to opportunistic Aspergillus parasites in honey bee larvae. J Invertebr Pathol 111:68–73
Fountain T, Hughes WOH (2011) Weaving resistance: silk and disease resistance in the weaver ant Polyrhachis dives. Insectes Soc 58:453–458
Gerardo NM, Jacobs SR, Currie CR, Mueller UG (2006) Ancient host-pathogen associations maintained by specificity of chemotaxis and antibiosis. PLoS Biol 4:1358–1363
Gillespie JP, Kanost MR, Trenczek T (1997) Biological mediators of insect immunity. Annu Rev Entomol 42:611–643
Graystock P, Hughes WOH (2011) Disease resistance in a weaver ant, Polyrhachis dives, and the role of antibiotic-producing glands. Behav Ecol Sociobiol 65:2319–2327
Haine ER, Moret Y, Siva-Jothy MT, Rolff J (2008) Antimicrobial defense and persistent infection in insects. Science 322:1257–1259
Hamilton C, Lejeune BT, Rosengaus RB (2011) Trophallaxis and prophylaxis: social immunity in the carpenter ant Camponotus pennsylvanicus. Biol Lett 7:89–92
Henry T, Iwen P, Hinrichs S (2000) Identification of Aspergillus species using internal transcribed spacer regions 1 and 2. J Clin Microbiol 38:1510–1515
Hölldobler B, Wilson EO (1990) The ants. Belknap Press, Cambridge
Hughes WOH, Eilenberg J, Boomsma JJ (2002) Trade-offs in group living: transmission and disease resistance in leaf-cutting ants. Proc R Soc Lond B 269:1811–1819
Hughes WOH, Petersen KS, Ugelvig LV, Pedersen D, Thomsen L, Poulsen M, Boomsma JJ (2004a) Density-dependence and within-host competition in a semelparous parasite of leaf-cutting ants. BMC Evol Biol 4:45–57
Hughes WOH, Thomsen L, Eilenberg J, Boomsma JJ (2004b) Diversity of entomopathogenic fungi near leaf-cutting ant nests in a neotropical forest, with particular reference to Metarhizium anisopliae var. anisopliae. J Invertebr Pathol 85:46–53
Hughes WOH, Pagliarini R, Madsen HB, Dijkstra MB, Boomsma JJ (2008) Antimicrobial defense shows an abrupt evolutionary transition in the fungus-growing ants. Evolution 62:1252–1257
Hughes WOH, Bot ANM, Boomsma JJ (2010) Caste-specific expression of genetic variation in the size of antibiotic-producing glands of leaf-cutting ants. Proc R Soc Lond B 277:609–615
Keller S, Kessler P, Schweizer C (2003) Distribution of insect pathogenic soil fungi in Switzerland with special reference to Beauveria brongniartii and Metharhizium anisopliae. BioControl 48:307–319
Konrad M, Vyleta ML, Theis FJ, Stock M, Tragust S, Klatt M, Drescher V, Marr C, Ugelvig LV, Cremer S (2012) Social transfer of pathogenic fungus promotes active immunisation in ant colonies. PLoS Biol 10:1–15
Krause J, Ruxton GD (2002) Living in groups. Oxford University Press, New York
Kuhn-Nentwig L (2003) Antimicrobial and cytolytic peptides of venomous arthropods. Cell Mol Life Sci 60:2651–2668
Lacerda FG, Della Lucia TMC, Pereira OL, Peternelli LA, Tótola MR (2010) Mortality of Atta sexdens rubropilosa (Hymenoptera: Formicidae) workers in contact with colony waste from different plant sources. Bull Entomol Res 100:99–103
Latgé J (1999) Aspergillus fumigatus and aspergillosis. Clin Microbiol Rev 12:310–350
Lavine MD, Strand MR (2002) Insect hemocytes and their role in immunity. Insect Biochem Mol Biol 32:1295–1309
Little AEF, Murakami T, Mueller UG, Currie CR (2006) Defending against parasites: fungus-growing ants combine specialized behaviours and microbial symbionts to protect their fungus gardens. Biol Lett 2:12–16
Mackintosh JA, Trimble JE, Beattie AJ, Veal DA, Jones MK, Karuso PH (1995) Antimicrobial mode of action of secretions from the metapleural gland of Myrmecia gulosa (Australian bull ant). Can J Microbiol 41:136–144
Mendonça ADL, da Silva CE, de Mesquita FLT, Campos RDS, Nascimento RR, Do XECPDA, Sant’Ana AEG (2009) Antimicrobial activities of components of the glandular secretions of leaf cutting ants of the genus Atta. Antonie Van Leeuwenhoek 95:295–303
Milner RJ, Staples JA, Lutton GG (1998) The selection of an isolate of the Hyphomycete fungus, Metarhizium anisopliae, for control of termites in Australia. Bio Control 247:240–247
Morelos-Juárez C, Walker TN, Lopes JFS, Hughes WOH (2010) Ant farmers practice proactive personal hygiene to protect their fungus crop. Curr Biol 20:553–554
Mueller U, Rehner S, Schultz T (1998) The evolution of agriculture in ants. Science 281:2034–2038
Pereira R, Stimac J (1997) Biocontrol options for urban pest ants. J Agric Entomol 14:231–248
Poulsen M, Bot ANM, Nielsen MG, Boomsma JJ (2002) Experimental evidence for the costs and hygienic significance of the antibiotic metapleural gland secretion in leaf-cutting ants. Behav Ecol Sociobiol 52:151–157
Poulsen M, Hughes WOH, Boomsma JJ (2006) Differential resistance and the importance of antibiotic production in Acromyrmex echinatior leaf-cutting ant castes towards the entomopathogenic fungus Aspergillus. Insectes Soc 53:349–355
Reber A, Chapuisat M (2011) Diversity, prevalence and virulence of fungal entomopathogens in colonies of the ant Formica selysi. Insectes Soc 59:231–239
Reber A, Chapuisat M (2012) No evidence for immune priming in ants exposed to a fungal pathogen. PloS one 7:1–6
Reber A, Purcell J, Buechel SD, Buri P, Chapuisat M (2011) The expression and impact of antifungal grooming in ants. J Evol Biol 24:954–964
Rosengaus RB, Maxmen AB, Coates LE, Traniello JFA (1998) Disease resistance: a benefit of sociality in the dampwood termite Zootermopsis angusticollis (Isoptera: Termopsidae). Behav Ecol Sociobiol 44:125–134
Rosengaus RB, Lefebvre ML, Traniello JFA (2000) Inhibition of fungal spore germination by Nasutitermes: evidence for a possible antiseptic role of soldier defensive secretions. J Chem Ecol 26:21–39
Rosengaus RB, Traniello JFA, Lefebvre ML, Maxmen AB (2004) Fungistatic activity of the sternal gland secretion of the dampwood termite Zootermopsis angusticollis. Insectes Soc 51:1–6
Schmid-Hempel P (1998) Parasites in social insects. Princeton University Press, Princeton
Storey GK, Meer RK, Vander BDG, McCoy CW (1991) Effect of fire ant (Solenopsis invicta) venom alkaloids on the in vitro germination and development of selected entomogenous fungi. J Invertebr Pathol 58:88–95
Stow A, Beattie A (2008) Chemical and genetic defences against disease in insect societies. Brain Behav Immun 22:1009–1013
Stow A, Briscoe D, Gillings M, Holley M, Smith S, Leys R, Silberbauer T, Turnbull C, Beattie A (2007) Antimicrobial defences increase with sociality in bees. Biol Lett 3:422–424
Sumner S, Hughes WOH, Boomsma JJ (2003) Evidence for differential selection and potential adaptive evolution in the worker caste of an inquiline social parasite. Behav Ecol Sociobiol 54:256–263
Tragust S, Mitteregger B, Barone V, Konrad M, Ugelvig LV, Cremer S (2013) Ants disinfect fungus-exposed brood by oral uptake and spread of their poison. Curr Biol 23:76–82
Traniello JFA, Rosengaus RB, Savoie K (2002) The development of immunity in a social insect: evidence for the group facilitation of disease resistance. Proc Natl Acad Sci U S A 99:6838–6842
Turillazzi S, Mastrobuoni G, Dani FR, Moneti G, Pieraccini G, la Marca G, Bartolucci G, Perito B, Lambardi D, Cavallini V, Dapporto L (2006) Dominulin A and B: two new antibacterial peptides identified on the cuticle and in the venom of the social paper wasp Polistes dominulus using MALDI-TOF, MALDI-TOF/TOF, and ESI-ion trap. J Am Soc Mass Spectrom 17:376–383
Turnbull C, Hoggard S, Gillings M, Palmer C, Stow A, Beattie D, Briscoe D, Smith S, Wilson P, Beattie A (2011) Antimicrobial strength increases with group size: implications for social evolution. Biol Lett 7:249–252
Turnbull C, Caravan H, Chapman T, Nipperess D, Dennison S, Schwarz M, Beattie A (2012) Antifungal activity in thrips soldiers suggests a dual role for this caste. Biol Lett 8:526–529
Ugelvig LV, Cremer S (2007) Social prophylaxis: group interaction promotes collective immunity in ant colonies. Curr Biol 17:1967–1971
Walker TN, Hughes WOH (2009) Adaptive social immunity in leaf-cutting ants. Biol Lett 5:446–448
Wilson-Rich N, Dres ST, Starks PT (2008) The ontogeny of immunity: development of innate immune strength in the honey bee (Apis mellifera). J Insect Physiol 54:1392–1399
Wilson-Rich N, Spivak M, Fefferman NH, Starks PT (2009) Genetic, individual, and group facilitation of disease resistance in insect societies. Annu Rev Entomol 54:405–423
Zelezetsky I, Pag U, Antcheva N, Sahl H-G, Tossi A (2005) Identification and optimization of an antimicrobial peptide from the ant venom toxin pilosulin. Arch Biochem Biophys 434:358–364
Zenghe W (1986) A preliminary study of the ant, Polyrhachis dives F. Smith. Sci Silvae Sin 4:15
Acknowledgments
We thank P. Chappell, C. Frost, R. Mitchell, and J. Parkinson for the technical assistance, V. Norman and two anonymous reviewers for their constructive comments which improved the manuscript, IBAMA for the permission to collect and export the leaf-cutting ant colonies, Martin Sebesta for providing the weaver ant colonies, and the Royal Society, BBSRC, and Leverhulme Foundation for the funding.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by D. Naug
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Details of treatment structure and subject assignment for Experiment 1. 120 leaf-cutting ants (a) were used in total, split between two equal cohorts. Each of the cohorts consisted of 20 ants from each of the three colonies, giving a total of 60 ants for each cohort. Within each cohort, ants from individual colonies were divided evenly into four treatment groups, consisting of five ants. This gave a total of 30 replicate ants per treatment across all colonies and cohorts. 160 weaver ants (b) were used in total, split between two equal cohorts. Each of the cohorts consisted of 40 ants from each of the two colonies, giving a total of 80 ants for each cohort. Within each cohort, ants from individual colonies were split evenly into four treatment groups, consisting of 10 ants. This gave a total of 40 replicate ants per treatment across all colonies and cohorts. (JPEG 186 kb)
Fig. S2
Details of treatment structure and subject assignment for Experiment 2. 120 leaf-cutting ants (a) were used in total, consisting of 40 ants from each of the three colonies. Ants from individual colonies were divided evenly into four treatment groups, consisting of 10 ants. This gave a total of 30 replicate ants per treatment across all colonies. Fifteen additional blank trials were conducted in the absence of any ant. 60 weaver ants (b) were used in total, consisting of 30 ants from each of the two colonies. Ants from individual colonies were divided evenly into two treatment groups, consisting of 15 ants each. This gave a total of 30 replicate ants per treatment across all colonies. Fifteen additional blank trials were conducted in the absence of any ant. (JPEG 140 kb)
Fig. S3
Results of experiment comparing brood-care behaviour and survival of nurse ants with blocked and unblocked glands. Both leaf-cutting ants (a; Wald = 5.6, d.f. = 1, p = 0.45) and weaver ants (b; Wald = 2.1, d.f. = 1, p = 0.15) showed no difference in survival of nurses with (open circles) or without (black circles) functional antimicrobial glands, whilst caring for brood treated with either Metarhizium parasite (solid lines) or control solution (dashed lines), over the course of the experiment. Additionally neither leaf-cutting ants (c) or weaver ants (d) showed any differences in the duration of time spent interacting with brood (U = 39, d.f. = 9, z = 0.84, p = 0.4, and U = 41.5, d.f. = 9, z = 0.64, p = 0.52, respectively), the incidences of contact with brood (U = 46, d.f. = 9, z = 0.36, p = 0.72, and U = 43, d.f. = 9, z = 0.54, p = 0.59, respectively), or incidences of brood-grooming (U = 49.5, d.f. = 9, z = 0.54, p = 0.96, and U = 46.5, d.f. = 9, z = 0.27, p = 0.79, respectively), between nurse ants with blocked (dark bars) and unblocked glands (light bars). (PNG 274 kb)
Rights and permissions
About this article
Cite this article
Tranter, C., Graystock, P., Shaw, C. et al. Sanitizing the fortress: protection of ant brood and nest material by worker antibiotics. Behav Ecol Sociobiol 68, 499–507 (2014). https://doi.org/10.1007/s00265-013-1664-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-013-1664-9