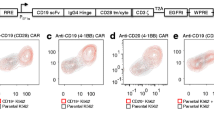
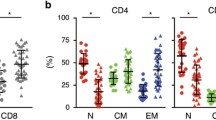
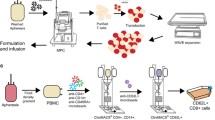
Abstract
The adoptive transfer of lymphocytes genetically engineered to express tumor-specific antigen receptors is a potent strategy to treat cancer patients. T lymphocyte subsets, such as naïve or central memory T cells, selected in vitro prior to genetic engineering have been extensively investigated in preclinical mouse models, where they demonstrated improved therapeutic efficacy. However, so far, this is challenging to realize in the clinical setting, since good manufacturing practices (GMP) procedures for complex cell sorting and genetic manipulation are limited. To be able to directly compare the immunological attributes and therapeutic efficacy of naïve (TN) and central memory (TCM) CD8+ T cells, we investigated clinical-scale procedures for their parallel selection and in vitro manipulation. We also evaluated currently available GMP-grade reagents for stimulation of T cell subsets, including a new type of anti-CD3/anti-CD28 nanomatrix. An optimized protocol was established for the isolation of both CD8+ TN cells (CD4−CD62L+CD45RA+) and CD8+ TCM (CD4−CD62L+CD45RA−) from a single patient. The highly enriched T cell subsets can be efficiently transduced and expanded to large cell numbers, sufficient for clinical applications and equivalent to or better than current cell and gene therapy approaches with unselected lymphocyte populations. The GMP protocols for selection of TN and TCM we reported here will be the basis for clinical trials analyzing safety, in vivo persistence and clinical efficacy in cancer patients and will help to generate a more reliable and efficacious cellular product.





Similar content being viewed by others
References
Restifo NP, Dudley ME, Rosenberg SA (2012) Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol 12:269–281
Brenner MK, Heslop HE (2010) Adoptive T cell therapy of cancer. Curr Opin Immunol 22:251–257
Johnson LA, Morgan RA, Dudley ME et al (2009) Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood 114:535–546
Morgan RA, Dudley ME, Wunderlich JR et al (2006) Cancer regression in patients after transfer of genetically engineered lymphocytes. Science 314:126–129
Robbins PF, Morgan RA, Feldman SA et al (2011) Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol 29:917–924
Parkhurst MR, Yang JC, Langan RC et al (2011) T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol Ther 19:620–626
Kochenderfer JN, Wilson WH, Janik JE et al (2010) Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood 116:4099–4102
Porter DL, Levine BL, Kalos M, Bagg A, June CH (2011) Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med 365:725–733
Chinnasamy D, Yu Z, Theoret MR et al (2010) Gene therapy using genetically modified lymphocytes targeting VEGFR-2 inhibits the growth of vascularized syngenic tumors in mice. J Clin Invest 120:3953–3968
Pule MA, Savoldo B, Myers GD et al (2008) Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med 14:1264–1270
Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA (2010) Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther 18:843–851
Lamers CH, Willemsen R, van Elzakker P et al (2011) Immune responses to transgene and retroviral vector in patients treated with ex vivo-engineered T cells. Blood 117:72–82
Johnson LA, Heemskerk B, Powell DJ Jr et al (2006) Gene transfer of tumor-reactive TCR confers both high avidity and tumor reactivity to nonreactive peripheral blood mononuclear cells and tumor-infiltrating lymphocytes. J Immunol 177:6548–6559
Gattinoni L, Powell DJ Jr, Rosenberg SA, Restifo NP (2006) Adoptive immunotherapy for cancer: building on success. Nat Rev Immunol 6:383–393
Zhou J, Shen X, Huang J, Hodes RJ, Rosenberg SA, Robbins PF (2005) Telomere length of transferred lymphocytes correlates with in vivo persistence and tumor regression in melanoma patients receiving cell transfer therapy. J Immunol 175:7046–7052
Hinrichs CS, Borman ZA, Cassard L et al (2009) Adoptively transferred effector cells derived from naive rather than central memory CD8+ T cells mediate superior antitumor immunity. Proc Natl Acad Sci USA 106:17469–17474
Berger C, Jensen MC, Lansdorp PM, Gough M, Elliott C, Riddell SR (2008) Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates. J Clin Invest 118:294–305
Jorritsma A, Schotte R, Coccoris M, de Witte MA, Schumacher TN (2011) Prospects and limitations of T cell receptor gene therapy. Curr Gene Ther 11:276–287
Lamers CH, van de Griend RJ, Braakman E et al (1992) Optimization of culture conditions for activation and large-scale expansion of human T lymphocytes for bispecific antibody-directed cellular immunotherapy. Int J Cancer 51:973–979
Levine BL, Mosca JD, Riley JL et al (1996) Antiviral effect and ex vivo CD4+ T cell proliferation in HIV-positive patients as a result of CD28 costimulation. Science 272:1939–1943
Bialer G, Horovitz-Fried M, Ya’acobi S, Morgan RA, Cohen CJ (2010) Selected murine residues endow human TCR with enhanced tumor recognition. J Immunol 184:6232–6241
Bunnell BA, Muul LM, Donahue RE, Blaese RM, Morgan RA (1995) High-efficiency retroviral-mediated gene transfer into human and nonhuman primate peripheral blood lymphocytes. Proc Natl Acad Sci USA 92:7739–7743
Feldman SA, Goff SL, Xu H et al (2011) Rapid production of clinical-grade gammaretroviral vectors in expanded surface roller bottles using a “modified” step-filtration process for clearance of packaging cells. Hum Gene Ther 22:107–115
Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A (1999) Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401:708–712
Huang J, Kerstann KW, Ahmadzadeh M et al (2006) Modulation by IL-2 of CD70 and CD27 expression on CD8+ T cells: importance for the therapeutic effectiveness of cell transfer immunotherapy. J Immunol. 176:7726–7735
Huang J, Khong HT, Dudley ME et al (2005) Survival, persistence, and progressive differentiation of adoptively transferred tumor-reactive T cells associated with tumor regression. J Immunother 28:258–267
Geginat J, Lanzavecchia A, Sallusto F (2003) Proliferation and differentiation potential of human CD8+ memory T-cell subsets in response to antigen or homeostatic cytokines. Blood 101:4260–4266
Ho WY, Blattman JN, Dossett ML, Yee C, Greenberg PD (2003) Adoptive immunotherapy: engineering T cell responses as biologic weapons for tumor mass destruction. Cancer Cell 3:431–437
Rosenberg SA (2012) Raising the bar: the curative potential of human cancer immunotherapy. Sci Transl Med 4:127ps8
Rosenberg SA, Yang JC, Sherry RM et al (2011) Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res 17:4550–4557
Gattinoni L, Klebanoff CA, Palmer DC et al (2005) Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J Clin Invest 115:1616–1626
Hinrichs CS, Borman ZA, Gattinoni L et al (2011) Human effector CD8+ T cells derived from naive rather than memory subsets possess superior traits for adoptive immunotherapy. Blood 17:808–814
Klebanoff CA, Gattinoni L, Torabi-Parizi P et al (2005) Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc Natl Acad Sci USA 102:9571–9576
Klebanoff CA, Finkelstein SE, Surman DR et al (2004) IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8+ T cells. Proc Natl Acad Sci USA 101:1969–1974
Wang X, Berger C, Wong CW, Forman SJ, Riddell SR, Jensen MC (2011) Engraftment of human central memory-derived effector CD8+ T cells in immunodeficient mice. Blood 117:1888–1898
Chapuis AG, Thompson JA, Margolin KA et al (2012) Transferred melanoma-specific CD8+ T cells persist, mediate tumor regression, and acquire central memory phenotype. Proc Natl Acad Sci USA 109:4592–4597
Besser MJ, Shapira-Frommer R, Treves AJ et al (2009) Minimally cultured or selected autologous tumor-infiltrating lymphocytes after a lympho-depleting chemotherapy regimen in metastatic melanoma patients. J Immunother 32:415–423
Dudley ME, Wunderlich JR, Shelton TE, Even J, Rosenberg SA (2003) Generation of tumor-infiltrating lymphocyte cultures for use in adoptive transfer therapy for melanoma patients. J Immunother 26:332–342
von Andrian UH, Mackay CR (2000) T-cell function and migration. Two sides of the same coin. N Engl J Med 343:1020–1034
Bannard O, Kraman M, Fearon D (2009) Pathways of memory CD8+ T-cell development. Eur J Immunol 39:2083–2087
Kaneko S, Mastaglio S, Bondanza A et al (2009) IL-7 and IL-15 allow the generation of suicide gene-modified alloreactive self-renewing central memory human T lymphocytes. Blood 113:1006–1015
Hinrichs CS, Spolski R, Paulos CM et al (2008) IL-2 and IL-21 confer opposing differentiation programs to CD8+ T cells for adoptive immunotherapy. Blood 111:5326–5333
Maus MV, Thomas AK, Leonard DG et al (2002) Ex vivo expansion of polyclonal and antigen-specific cytotoxic T lymphocytes by artificial APCs expressing ligands for the T-cell receptor, CD28 and 4-1BB. Nat Biotechnol 20:143–148
Martin-Orozco N, Muranski P, Chung Y et al (2009) T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity 31:787–798
Hunder NN, Wallen H, Cao J et al (2008) Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N Engl J Med 358:2698–2703
Acknowledgments
This work was supported by grants from ATTRACT European Consortium (FP7-PEOPLE-ITN-2008, Grant Agreement Number 238778); Anna Casati was recipient of a Marie Curie fellowship.
Conflict of interest
Mario Assenmacher is employee of Miltenyi Biotec GmbH and Alexander Scheffold works as a consultant for Miltenyi Biotec GmbH. All other authors do not have any conflicts to disclose.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Casati, A., Varghaei-Nahvi, A., Feldman, S.A. et al. Clinical-scale selection and viral transduction of human naïve and central memory CD8+ T cells for adoptive cell therapy of cancer patients. Cancer Immunol Immunother 62, 1563–1573 (2013). https://doi.org/10.1007/s00262-013-1459-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-013-1459-x