Abstract
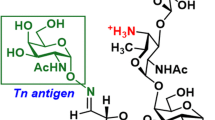
The Tn antigen (GalNAcα-O-Ser/Thr) is a well-established tumor-associated marker which represents a good target for the design of anti-tumor vaccines. Several studies have established that the binding of some anti-Tn antibodies could be affected by the density of Tn determinant or/and by the amino acid residues neighboring O-glycosylation sites. In the present study, using synthetic Tn-based vaccines, we have generated a panel of anti-Tn monoclonal antibodies. Analysis of their binding to various synthetic glycopeptides, modifying the amino acid carrier of the GalNAc(*) (Ser* vs Thr*), showed subtle differences in their fine specificities. We found that the recognition of these glycopeptides by some of these MAbs was strongly affected by the Tn backbone, such as a S*S*S* specific MAb (15G9) which failed to recognize a S*T*T* or a T*T*T* structure. Different binding patterns of these antibodies were also observed in FACS and Western blot analysis using three human cancer cell lines (MCF-7, LS174T and Jurkat). Importantly, an immunohistochemical analysis of human tumors (72 breast cancer and 44 colon cancer) showed the existence of different recognition profiles among the five antibodies evaluated, demonstrating that the aglyconic part of the Tn structure (Ser vs Thr) plays a key role in the anti-Tn specificity for breast and colon cancer detection. This new structural feature of the Tn antigen could be of important clinical value, notably due to the increasing interest of this antigen in anticancer vaccine design as well as for the development of anti-Tn antibodies for in vivo diagnostic and therapeutic strategies.







Similar content being viewed by others
References
Ohtsubo K, Marth JD (2006) Glycosylation in cellular mechanisms of health and disease. Cell 126:855–867. doi:10.1016/j.cell.2006.08.019
Baldus SE, Engelmann K, Hanisch FG (2004) MUC1 and the MUCs: a family of human mucins with impact in cancer biology. Crit Rev Clin Lab Sci 41:189–231. doi:10.1080/10408360490452040
Kufe D (2009) Mucins in cancer: function, prognosis and therapy. Nat Rev Cancer 9:874–885. doi:10.1038/nrc2761
Tsuboi S, Hatakeyama S, Ohyama C, Fukuda M (2012) Two opposing roles of O-glycans in tumor metastasis. Trends Mol Med 18:224–232. doi:10.1016/j.molmed.2012.02.001
Rabinovich GA, van Kooyk Y, Cobb BA (2012) Glycobiology of immune responses. Ann N Y Acad Sci 1253:1–15. doi:10.1111/j.1749-6632.2012.06492.x
Ten Hagen KG, Fritz TA, Tabak LA (2003) All in the family: the UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferases. Glycobiology 13:1R–16R. doi:10.1093/glycob/cwg007
Brockhausen I (2006) Mucin-type O-glycans in human colon and breast cancer: glycodynamics and functions. EMBO Rep 7:599–604. doi:10.1038/sj.embor.7400705
Ju T, Cummings RD (2002) A unique molecular chaperone Cosmc required for activity of the mammalian core 1 beta 3-galactosyltransferase. Proc Natl Acad Sci USA 99:16613–16618. doi:10.1073/pnas.262438199
Mi R, Song L, Wang Y, Ding X, Zeng J, Lehoux S, Aryal RP, Wang J, Crew VK, van Die I, Chapman AB, Cummings RD, Ju T (2012) Epigenetic silencing of the chaperone Cosmc in human leukocytes expressing tn antigen. J Biol Chem 287:41523–41533. doi:0.1074/jbc.M112.371989
Schietinger A, Philip M, Yoshida B, Azadi P, Liu H, Meredith S, Schreiber H (2006) A mutant chaperone converts a wild-type protein into a tumor-specific antigen. Science 314:304–308. doi:10.1126/science.1129200
Ju T, Lanneau GS, Gautam T, Wang Y, Xia B, Stowell SR, Willard MT, Wang W, Xia JY, Zuna RE, Laszik Z, Benbrook DM, Hanigan MH, Cummings RD (2008) Human tumor antigens Tn and sialyl Tn arise from mutations in Cosmc. Cancer Res 68:1636–1646. doi:10.1158/0008-5472.CAN-07-2345
Freire T, Bay S, von Mensdorff-Pouilly S, Osinaga E (2005) Molecular basis of incomplete O-glycan synthesis in breast cancer cells: putative role of MUC6 in Tn antigen expression. Cancer Res 65:7880–7887. doi:10.1158/0008-5472.CAN-04-3746
Springer GF (1984) T and Tn, general carcinoma autoantigens. Science 224:1198–1206. doi:10.1126/science.6729450
Cao Y, Merling A, Karsten U, Goletz S, Punzel M, Kraft R, Butschak G, Schwartz-Albiez R (2008) Expression of CD175 (Tn), CD175 s (sialosyl-Tn) and CD176 (Thomsen-Friedenreich antigen) on malignant human hematopoietic cells. Int J Cancer 123:89–99. doi:10.1002/ijc.23493
Itzkowitz SH, Bloom EJ, Lau TS, Kim YS (1992) Mucin associated Tn and sialosyl-Tn antigen expression in colorectal polyps. Gut 33:518–523. doi:10.136/gut.33.4.518
Babino A, Oppezzo P, Bianco S, Barrios E, Berois N, Navarrete H, Osinaga E (2000) Tn antigen is a pre-cancerous biomarker in breast tissue and serum in N-nitrosomethylurea-induced rat mammary carcinogenesis. Int J Cancer 86:753–759. doi:10.1002/(SICI)1097-0215(20000615)86:6<753::AID-IJC1>3.0.CO;2-#
Springer GF (1997) Immunoreactive T and Tn epitopes in cancer diagnosis, prognosis, and immunotherapy. J Mol Med 75:594–602. doi:10.1007/s001090050144
Lo-Man R, Vichier-Guerre S, Perraut R, Dériaud E, Huteau V, BenMohamed L, Diop OM, Livingston PO, Bay S, Leclerc C (2004) A fully synthetic therapeutic vaccine candidate targeting carcinoma-associated Tn carbohydrate antigen induces tumor-specific antibodies in nonhuman primates. Cancer Res 64:4987–4994. doi:10.1158/0008-5472.CAN-04-0252
Tarp MA, Clausen H (2008) Mucin-type O-glycosylation and its potential use in drug and vaccine development. Biochim Biophys Acta 1780:546–563. doi:10.1016/j.bbagen.2007.09.010
Ju T, Otto VI, Cummings RD (2011) The Tn antigen—structural simplicity and biological complexity. Angew Chem Int Ed Engl 50:1770–1791. doi:10.1002/anie.201002313
Manimala JC, Li Z, Jain A, VedBrat S, Gildersleeve JC (2005) Carbohydrate array analysis of anti-Tn antibodies and lectins reveals unexpected specificities: implications for diagnostic and vaccine development. ChemBioChem 6:2229–2241
Huang J, Byrd JC, Siddiki B, Yuan M, Lau E, Kim YS (1992) Monoclonal antibodies against partially deglycosylated colon cancer mucin that recognize Tn antigen. Dis Markers 10:81–94
Itzkowitz S, Kjeldsen T, Friera A, Hakomori S, Yang US, Kim YS (1991) Expression of Tn, sialosyl Tn, and T antigens in human pancreas. Gastroenterology 100:1691–1700
Ching CK, Holmes SW, Holmes GK, Long RG (1994) Blood-group sialyl-Tn antigen is more specific than Tn as a tumor marker in the pancreas. Pancreas 9:698–702
Cao Y, Stosiek P, Springer GF, Karsten U (1996) Thomsen-Friedenreich-related carbohydrate antigens in normal adult human tissues: a systematic and comparative study. Histochem Cell Biol 106:197–207. doi:10.1007/BF02484401
Kawaguchi T, Takazawa H, Imai S, Morimoto J, Watanabe T, Kanno M, Igarashi S (2006) Expression of Vicia villosa agglutinin (VVA)-binding glycoprotein in primary breast cancer cells in relation to lymphatic metastasis: is atypical MUC1 bearing Tn antigen a receptor of VVA? Breast Cancer Res Treat 98:31–43. doi:10.1007/s10549-005-9115-6
Grinstead JS, Koganty RR, Krantz MJ, Longenecker BM, Campbell AP (2002) Effect of glycosylation on MUC1 humoral immune recognition: NMR studies of MUC1 glycopeptide-antibody interactions. Biochemistry 41:9946–9961. doi:10.1021/bi012176z
Medeiros A, Bianchi S, Calvete JJ, Balter H, Bay S, Robles A, Cantacuzène D, Nimtz M, Alzari PM, Osinaga E (2000) Biochemical and functional characterization of the Tn-specific lectin from Salvia sclarea seeds. Eur J Biochem 267:1434–1440. doi:10.1046/j.1432-1327.2000.01141.x
Osinaga E, Bay S, Tello D, Babino A, Pritsch O, Assemat K, Cantacuzene D, Nakada H, Alzari P (2000) Analysis of the fine specificity of Tn-binding proteins using synthetic glycopeptide epitopes and a biosensor based on surface plasmon resonance spectrospcopy. FEBS Lett 469:24–28. doi:10.1016/S0014-5793(00)01248-5
Nakada H, Inoue M, Numata Y, Tanaka N, Funakoshi I, Fukui S, Mellors A, Yamashina I (1993) Epitopic structure of Tn glycophorin A for an anti-Tn antibody (MLS 128). Proc Natl Acad Sci USA 90:2495–2499. doi:10.1073/pnas.90.6.2495
Reis CA, Sorensen T, Mandel U, David L, Mirgorodskaya E, Roepstorff P, Kihlberg J, Hansen JE, Clausen H (1998) Development and characterization of an antibody directed to an alpha-N-acetyl-D-galactosamine glycosylated MUC2 peptide. Glycoconj J 15:51–62. doi:10.1023/A:1006939432665
Kuduk S, Schwarz J, Chen XT, Glunz P, Sames D, Ragupathi G, Livingston PO, Danishefsky S (1998) Synthetic and immunological studies of clustered modes of mucin-related Tn and TF O-linked antigens: the preparation of a glycopeptide-based vaccines for clinical trials against prostate cancer. J Am Chem Soc 120:12474–12485
Bay S, Lo-Man R, Osinaga E, Nakada H, Leclerc C, Cantacuzène D (1997) Preparation of a multiple antigen glycopeptide (MAG) carrying the Tn antigen. A possible approach to a synthetic carbohydrate vaccine. J Peptide Res 49:620–625
Lo-Man R, Bay S, Vichier-Guerre S, Deriaud E, Cantacuzene D, Leclerc C (1999) A fully synthetic immunogen carrying a carcinoma-associated carbohydrate for active specific immunotherapy. Cancer Res 59:1520–1524
Lo-Man R, Vichier-Guerre S, Bay S, Deriaud E, Cantacuzene D, Leclerc C (2001) Anti-tumor immunity provided by a synthetic multiple antigenic glycopeptide displaying a tri-Tn glycotope. J Immunol 166:2849–2854
Pancino G, Osinaga E, Vorauher W, Kakouche A, Mistro D, Charpin C, Roseto A (1990) Production of a monoclonal antibody as immunohistochemical marker on paraffin embedded tissues using a new immunization method. Hybridoma 9:389–395
Vichier-Guerre S, Lo-Man R, Bay S, Deriaud E, Nakada H, Leclerc C, Cantacuzène D (2000) Short synthetic glycopeptides successfully induce antibody responses to carcinoma-associated Tn antigen. J Peptide Res 55:173–180. doi:10.1034/j.1399-3011.2000.00167.x
Kononen J, Bubendorf L, Kallioniemi A, Bärlund M, Schraml P, Leighton S, Torhorst J, Mihatsch MJ, Sauter G, Kallioniemi OP (1998) Tissue microarrays for high-throughput profiling of tumor specimens. Nat Med 4:844–847. doi:10.1038/nm0798-844
Camp RL, Charette LA, Rimm DL (2000) Validation of tissue microarray technology in breast carcinoma. Lab Invest 80:1943–1949. doi:10.1038/labinvest.3780204
Li Q, Anver MR, Butcher DO, Gildersleeve JC (2009) Resolving conflicting data on expression of the Tn antigen and implications for clinical trials with cancer vaccines. Mol Cancer Ther 8:971–979. doi:10.1158/1535-7163.MCT-08-0934
Reddish MA, Jackson L, Koganty RR, Qiu D, Hong W, Longenecker BM (1997) Specificities of anti-sialyl-Tn and anti-Tn monoclonal antibodies generated using novel clustered synthetic glycopeptide epitopes. Glycoconj J 14:549–560
Corzana F, Busto JH, Jiménez-Osés G, García de Luis M, Asensio JL, Jiménez-Barbero J, Peregrina JM, Avenoza A (2007) Serine versus threonine glycosylation: the methyl group causes a drastic alteration on the carbohydrate orientation and on the surrounding water shell. J Am Chem Soc 129:9458–9467. doi:10.1021/ja072181b
Corzana F, Busto JH, García de Luis M, Jiménez-Barbero J, Avenoza A, Peregrina JM (2009) The nature and sequence of the amino acid aglycone strongly modulates the conformation and dynamics effects of Tn antigen’s clusters. Chemistry 15:3863–3874. doi:10.1002/chem.200801777
Tachibana Y, Fletcher GL, Fujitani N, Tsuda S, Monde K, Nishimura SI (2004) Antifreeze glycoproteins: elucidation of the structural motifs that are essential for antifreeze activity. Angew Chem Int Ed 43:856–862. doi:10.1002/anie.200353110
Blixt O, Lavrova OI, Mazurov DV, Cló E, Kracun SK, Bovin NV, Filatov AV (2012) Analysis of Tn antigenicity with a panel of new IgM and IgG1 monoclonal antibodies raised against leukemic cells. Glycobiology 22:529–542. doi:10.1093/glycob/cwr178
Thanka Christlet TH, Veluraja K (2001) Database analysis of O-glycosylation sites in proteins. Biophys J 80:952–960
Elhammer AP, Kézdy FJ, Kurosaka A (1999) The acceptor specificity of UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferases. Glycoconj J 16:171–180. doi:10.1023/A:1026465232149
Von Mensdorff-Pouilly S, Petrakou E, Kenemans P, Van Uffelen K, Verstraeten AA, Snijdewint FG, Van Kamp GJ, Schol DJ, Reis CA, Price MR, Livingston PO, Hilgers J (2000) Reactivity of natural and induced human antibodies to MUC1 mucin with MUC1 peptides and N-acetylgalactosamine (GalNAc) peptides. Int J Cancer 86:702–712. doi:10.1002/(SICI)1097-0215(20000601)86:5<702:AID-IJC16>3.0.CO;2-1
Smorodin EP, Kurtenkov OA, Sergeyev BL, Kodar KE, Chuzmarov VI, Afanasyev VP (2008) Postoperative change of anti-Thomsen-Friedenreich and Tn IgG level: the follow-up study of gastrointestinal cancer patients. World J Gastroenterol 14:4352–4358. doi:10.3748/wjg.14.4352
Oyelaran O, Li Q, Farnsworth D, Gildersleeve JC (2009) Microarrays with varying carbohydrate density reveal distinct subpopulations of serum antibodies. J Proteome Res 8:3529–3538. doi:10.1021/pr9002245
Matsukita S, Nomoto M, Kitajima S, Tanaka S, Goto M, Irimura T, Kim YS, Sato E, Yonezawa S (2003) Expression of mucins (MUC1, MUC2, MUC5AC and MUC6) in mucinous carcinoma of the breast: comparison with invasive ductal carcinoma. Histopathology 42:26–36. doi:10.1046/j.1365-2559.2003.01530.x
Pereira MB, Dias AJ, Reis CA, Schmitt FC (2001) Immunohistochemical study of the expression of MUC5AC and MUC6 in breast carcinomas and adjacent breast tissues. J Clin Pathol 54:210–213. doi:10.1136/jcp.54.3.210
Berois N, Varangot M, Sóñora C, Zarantonelli L, Pressa C, Laviña R, Porchet N, Aubert JP, Osinaga E (2003) Detection of bone marrow-disseminated breast cancer cells using a nested RT-PCR assay of MUC5B mRNA. Int J Cancer 103:550–555. doi:10.1002/ijc.10853
de Bolòs C, Gumà M, Barranco C, Garrido M, Kim YS, Real FX (1998) MUC6 expression in breast tissues and cultured cells: abnormal expression in tumors and regulation by steroid hormones. Int J Cancer 77:193–199. doi:10.1002/(SICI)1097-0215(19980717)77:2<193:AID-IJC4>3.0.CO;2-L
Byrd JC, Bresalier RS (2004) Mucins and mucin binding proteins in colorectal cancer. Cancer Metastasis Rev 23:77–99. doi:10.1023/A:1025815113599
Welinder C, Baldetorp B, Borrebaeck C, Fredlund BM, Jansson B (2011) A new murine IgG1 anti-Tn monoclonal antibody with in vivo anti-tumor activity. Glycobiology 21:1097–1107. doi:10.1093/glycob/cwr048
Ando H, Matsushita T, Wakitani M, Sato T, Kodama-Nishida S, Shibata K, Shitara K, Ohta S (2008) Mouse-human chimeric anti-Tn IgG1 induced anti-tumor activity against Jurkat cells in vitro and in vivo. Biol Pharm Bull 31:1739–1744. doi:10.1248/bpb.31.1739
Hubert P, Heitzmann A, Viel S, Nicolas A, Sastre-Garau X, Oppezzo P, Pritsch O, Osinaga E, Amigorena S (2011) Antibody-dependent cell cytotoxicity synapses form in mice during tumor-specific antibody immunotherapy. Cancer Res 71:5134–5143. doi:10.1158/0008-5472.CAN-10-4222
Lavrsen K, Madsen CB, Rasch MG, Woetmann A, Odum N, Mandel U, Clausen H, Pedersen AE, Wandall HH (2012) Aberrantly glycosylated MUC1 is expressed on the surface of breast cancer cells and a target for antibody-dependent cell-mediated cytotoxicity. Glycoconj J. doi:10.1007/s10719-012-9437-7
Danussi C, Coslovi A, Campa C, Mucignat MT, Spessotto P, Uggeri F, Paoletti S, Colombatti A (2009) A newly generated functional antibody identifies Tn antigen as a novel determinant in the cancer cell-lymphatic endothelium interaction. Glycobiology 19:1056–1067. doi:10.1093/glycob/cwp085
Morita N, Yajima Y, Asanuma H, Nakada H, Fujita-Yamaguchi Y (2009) Inhibition of cancer cell growth by anti-Tn monoclonal antibody MLS128. Biosci Trends 3:32–37
Acknowledgments
This work was supported by grants from Programmes Transversaux de Recherche (PTR, Institut Pasteur, Paris, France) and ECOS France-Uruguay Program to Eduardo Osinaga, Sylvie Bay and Claude Leclerc and from the Ligue Nationale Contre le Cancer (Equipe Labellisée 2011) and Banque Privée Européenne to Claude Leclerc, and Programa Grupos de Investigación (CSIC, Universidad de la República, Uruguay) to Eduardo Osinaga and Otto Pritsch.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mazal, D., Lo-Man, R., Bay, S. et al. Monoclonal antibodies toward different Tn-amino acid backbones display distinct recognition patterns on human cancer cells. Implications for effective immuno-targeting of cancer. Cancer Immunol Immunother 62, 1107–1122 (2013). https://doi.org/10.1007/s00262-013-1425-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-013-1425-7