Abstract
Purpose
Quantification of tumour heterogeneity in PET images has recently gained interest, but has been shown to be dependent on image reconstruction. This study aimed to evaluate the impact of the EANM/EARL accreditation program on selected 18F-FDG heterogeneity metrics.
Methods
To carry out our study, we prospectively analysed 71 tumours in 60 biopsy-proven lung cancer patient acquisitions reconstructed with unfiltered point spread function (PSF) positron emission tomography (PET) images (optimised for diagnostic purposes), PSF-reconstructed images with a 7-mm Gaussian filter (PSF7) chosen to meet European Association of Nuclear Medicine (EANM) 1.0 harmonising standards, and EANM Research Ltd. (EARL)-compliant ordered subset expectation maximisation (OSEM) images. Delineation was performed with fuzzy locally adaptive Bayesian (FLAB) algorithm on PSF images and reported on PSF7 and OSEM ones, and with a 50 % standardised uptake values (SUV)max threshold (SUVmax50%) applied independently to each image. Robust and repeatable heterogeneity metrics including 1st-order [area under the curve of the cumulative histogram (CHAUC)], 2nd-order (entropy, correlation, and dissimilarity), and 3rd-order [high-intensity larger area emphasis (HILAE) and zone percentage (ZP)] textural features (TF) were statistically compared.
Results
Volumes obtained with SUVmax50% were significantly smaller than FLAB-derived ones, and were significantly smaller in PSF images compared to OSEM and PSF7 images. PSF-reconstructed images showed significantly higher SUVmax and SUVmean values, as well as heterogeneity for CHAUC, dissimilarity, correlation, and HILAE, and a wider range of heterogeneity values than OSEM images for most of the metrics considered, especially when analysing larger tumours. Histological subtypes had no impact on TF distribution. No significant difference was observed between any of the considered metrics (SUV or heterogeneity features) that we extracted from OSEM and PSF7 reconstructions. Furthermore, the distributions of TF for OSEM and PSF7 reconstructions according to tumour volumes were similar for all ranges of volumes.
Conclusion
PSF reconstruction with Gaussian filtering chosen to meet harmonising standards resulted in similar SUV values and heterogeneity information as compared to OSEM images, which validates its use within the harmonisation strategy context. However, unfiltered PSF-reconstructed images also showed higher heterogeneity according to some metrics, as well as a wider range of heterogeneity values than OSEM images for most of the metrics considered, especially when analysing larger tumours. This suggests that, whenever available, unfiltered PSF images should also be exploited to obtain the most discriminative quantitative heterogeneity features.






Similar content being viewed by others
References
Boellaard R. Standards for PET image acquisition and quantitative data analysis. J Nucl Med. 2009;50 Suppl 1:11S–20S. doi:10.2967/jnumed.108.057182.
Boellaard R. Methodological aspects of multicenter studies with quantitative PET. Methods Mol Biol. 2011;727:335–49. doi:10.1007/978-1-61779-062-1_18.
Boellaard R. Mutatis mutandis: harmonize the standard! J Nucl Med. 2012;53:1–3. doi:10.2967/jnumed.111.094763.
American College of Radiology, Nuclear Medicine and PET accreditation program http://www.acraccreditation.org/Modalities/Nuclear-Medicine-and-PET.
European Association of Nuclear Medicine. EARL FDG-PET/CT accreditation. 2015. http://earl.eanm.org/cms/website.php?id=/en/projects/fdg_pet_ct_accreditation.htm.
The Society of Nuclear Medicine and Molecular Imaging Clinical trials network (SNMMI-CTN). Scanner validation program. http://www.snmmi.org/Research/Content.aspx?ItemNumber=5482&navItemNumber=6834.
Makris NE, Huisman MC, Kinahan PE, Lammertsma AA, Boellaard R. Evaluation of strategies towards harmonization of FDG PET/CT studies in multicentre trials: comparison of scanner validation phantoms and data analysis procedures. Eur J Nucl Med Mol Imaging. 2013;40:1507–15. doi:10.1007/s00259-013-2465-0.
Panin VY, Kehren F, Michel C, Casey M. Fully 3-D PET reconstruction with system matrix derived from point source measurements. IEEE Trans Med Imaging. 2006;25:907–21.
Teoh EJ, McGowan DR, Macpherson RE, Bradley KM, Gleeson FV. Phantom and clinical evaluation of the Bayesian penalized likelihood reconstruction algorithm Q.Clear on an LYSO PET/CT system. J Nucl Med. 2015. doi:10.2967/jnumed.115.159301.
Bellevre D, Blanc Fournier C, Switsers O, Dugue AE, Levy C, Allouache D, et al. Staging the axilla in breast cancer patients with 18F-FDG PET: how small are the metastases that we can detect with new generation clinical PET systems? Eur J Nucl Med Mol Imaging. 2014;41:1103–12. doi:10.1007/s00259-014-2689-7.
Boellaard R, Delgado-Bolton R, Oyen WJ, Giammarile F, Tatsch K, Eschner W, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;42:328–54. doi:10.1007/s00259-014-2961-x.
Lasnon C, Desmonts C, Quak E, Gervais R, Do P, Dubos-Arvis C, et al. Harmonizing SUVs in multicentre trials when using different generation PET systems: prospective validation in non-small cell lung cancer patients. Eur J Nucl Med Mol Imaging. 2013;40:985–96. doi:10.1007/s00259-013-2391-1.
Quak E, Le Roux PY, Hofman MS, Robin P, Bourhis D, Callahan J, et al. Harmonizing FDG PET quantification while maintaining optimal lesion detection: prospective multicentre validation in 517 oncology patients. Eur J Nucl Med Mol Imaging. 2015. doi:10.1007/s00259-015-3128-0.
Mertens J, Dobbeleir A, Ham H, D’Asseler Y, Goethals I, Van de Wiele C. Standardized added metabolic activity (SAM): a partial volume independent marker of total lesion glycolysis in liver metastases. Eur J Nucl Med Mol Imaging. 2012;39:1441–8. doi:10.1007/s00259-012-2166-0.
Visvikis D, Hatt M, Tixier F, Cheze Le Rest C. The age of reason for FDG PET image-derived indices. Eur J Nucl Med Mol Imaging. 2012;39:1670–2. doi:10.1007/s00259-012-2239-0.
El Naqa I, Grigsby P, Apte A, Kidd E, Donnelly E, Khullar D, et al. Exploring feature-based approaches in PET images for predicting cancer treatment outcomes. Pattern Recogn. 2009;42:1162–71. doi:10.1016/j.patcog.2008.08.011.
Galavis PE, Hollensen C, Jallow N, Paliwal B, Jeraj R. Variability of textural features in FDG PET images due to different acquisition modes and reconstruction parameters. Acta Oncol. 2010;49:1012–6. doi:10.3109/0284186X.2010.498437.
Yan J, Chu-Shern JL, Loi HY, Khor LK, Sinha AK, Quek ST, et al. Impact of image reconstruction settings on texture features in 18F-FDG PET. J Nucl Med. 2015;56:1667–73. doi:10.2967/jnumed.115.156927.
Hatt M, Majdoub M, Vallieres M, Tixier F, Le Rest CC, Groheux D, et al. 18F-FDG PET uptake characterization through texture analysis: investigating the complementary nature of heterogeneity and functional tumor volume in a multi-cancer site patient cohort. J Nucl Med. 2015;56:38–44. doi:10.2967/jnumed.114.144055.
Calais J, Dubray B, Nkhali L, Thureau S, Lemarignier C, Modzelewski R, et al. High FDG uptake areas on pre-radiotherapy PET/CT identify preferential sites of local relapse after chemoradiotherapy for locally advanced oesophageal cancer. Eur J Nucl Med Mol Imaging. 2015;42:858–67. doi:10.1007/s00259-015-3004-y.
Sauter AW, Schwenzer N, Divine MR, Pichler BJ, Pfannenberg C. Image-derived biomarkers and multimodal imaging strategies for lung cancer management. Eur J Nucl Med Mol Imaging. 2015;42:634–43. doi:10.1007/s00259-014-2974-5.
Takeuchi S, Khiewvan B, Fox PS, Swisher SG, Rohren EM, Bassett Jr RL, et al. Impact of initial PET/CT staging in terms of clinical stage, management plan, and prognosis in 592 patients with non-small-cell lung cancer. Eur J Nucl Med Mol Imaging. 2014;41:906–14. doi:10.1007/s00259-013-2672-8.
Vera P, Mezzani-Saillard S, Edet-Sanson A, Menard JF, Modzelewski R, Thureau S, et al. FDG PET during radiochemotherapy is predictive of outcome at 1 year in non-small-cell lung cancer patients: a prospective multicentre study (RTEP2). Eur J Nucl Med Mol Imaging. 2014;41:1057–65. doi:10.1007/s00259-014-2687-9.
Fried DV, Mawlawi O, Zhang L, Fave X, Zhou S, Ibbott G, et al. Stage III non-small cell lung cancer: prognostic value of FDG PET quantitative imaging features combined with clinical prognostic factors. Radiology. 2016;278:214–22. doi:10.1148/radiol.2015142920.
Lovinfosse P, Janvary ZL, Coucke P, Jodogne S, Bernard C, Hatt M, et al. FDG PET/CT texture analysis for predicting the outcome of lung cancer treated by stereotactic body radiation therapy. Eur J Nucl Med Mol Imaging. 2016. doi:10.1007/s00259-016-3314-8.
Aide N, Desmonts C, Beauregard JM, Beyer T, Kinross K, Roselt P, et al. High throughput static and dynamic small animal imaging using clinical PET/CT: potential preclinical applications. Eur J Nucl Med Mol Imaging. 2010;37:991–1001. doi:10.1007/s00259-009-1352-1.
Hatt M, Cheze le Rest C, Turzo A, Roux C, Visvikis D. A fuzzy locally adaptive Bayesian segmentation approach for volume determination in PET. IEEE Trans Med Imaging. 2009;28:881–93. doi:10.1109/TMI.2008.2012036.
Hatt M, Cheze Le Rest C, Albarghach N, Pradier O, Visvikis D. PET functional volume delineation: a robustness and repeatability study. Eur J Nucl Med Mol Imaging. 2011;38:663–72. doi:10.1007/s00259-010-1688-6.
Hatt M, Cheze-Le Rest C, Aboagye EO, Kenny LM, Rosso L, Turkheimer FE, et al. Reproducibility of 18F-FDG and 3′-deoxy-3′-18F-fluorothymidine PET tumor volume measurements. J Nucl Med. 2010;51:1368–76. doi:10.2967/jnumed.110.078501.
Hatt M, Cheze le Rest C, Descourt P, Dekker A, De Ruysscher D, Oellers M, et al. Accurate automatic delineation of heterogeneous functional volumes in positron emission tomography for oncology applications. Int J Radiat Oncol Biol Phys. 2010;77:301–8. doi:10.1016/j.ijrobp.2009.08.018.
Arens AI, Troost EG, Hoeben BA, Grootjans W, Lee JA, Gregoire V, et al. Semiautomatic methods for segmentation of the proliferative tumour volume on sequential FLT PET/CT images in head and neck carcinomas and their relation to clinical outcome. Eur J Nucl Med Mol Imaging. 2014;41:915–24. doi:10.1007/s00259-013-2651-0.
van Velden FH, Cheebsumon P, Yaqub M, Smit EF, Hoekstra OS, Lammertsma AA, et al. Evaluation of a cumulative SUV-volume histogram method for parameterizing heterogeneous intratumoural FDG uptake in non-small cell lung cancer PET studies. Eur J Nucl Med Mol Imaging. 2011;38:1636–47. doi:10.1007/s00259-011-1845-6.
Hatt M, Tixier F, Cheze Le Rest C, Pradier O, Visvikis D. Robustness of intratumour (1)(8)F-FDG PET uptake heterogeneity quantification for therapy response prediction in oesophageal carcinoma. Eur J Nucl Med Mol Imaging. 2013;40:1662–71. doi:10.1007/s00259-013-2486-8.
Tixier F, Hatt M, Le Rest CC, Le Pogam A, Corcos L, Visvikis D. Reproducibility of tumor uptake heterogeneity characterization through textural feature analysis in 18F-FDG PET. J Nucl Med. 2012;53:693–700. doi:10.2967/jnumed.111.099127.
Vallieres M, Freeman CR, Skamene SR, El Naqa I. A radiomics model from joint FDG-PET and MRI texture features for the prediction of lung metastases in soft-tissue sarcomas of the extremities. Phys Med Biol. 2015;60:5471–96. doi:10.1088/0031-9155/60/14/5471.
Webb AG. Introduction to biomedical imaging. Wiley-IEEE Press; 2003.
van Velden FH, Kramer GM, Frings V, Nissen IA, Mulder ER, de Langen AJ, et al. Repeatability of radiomic features in non-small-cell lung cancer [F]FDG-PET/CT studies: impact of reconstruction and delineation. Mol Imaging Biol Off Publ Acad Mol Imaging. 2016. doi:10.1007/s11307-016-0940-2.
Nyflot MJ, Yang F, Byrd D, Bowen SR, Sandison GA, Kinahan PE. Quantitative radiomics: impact of stochastic effects on textural feature analysis implies the need for standards. J Med Imaging (Bellingham). 2015;2:041002. doi:10.1117/1.JMI.2.4.041002.
Sunderland JJ, Christian PE. Quantitative PET/CT scanner performance characterization based upon the society of nuclear medicine and molecular imaging clinical trials network oncology clinical simulator phantom. J Nucl Med. 2015;56:145–52. doi:10.2967/jnumed.114.148056.
Groheux D, Majdoub M, Tixier F, Le Rest CC, Martineau A, Merlet P, et al. Do clinical, histological or immunohistochemical primary tumour characteristics translate into different (18)F-FDG PET/CT volumetric and heterogeneity features in stage II/III breast cancer? Eur J Nucl Med Mol Imaging. 2015;42:1682–91. doi:10.1007/s00259-015-3110-x.
Ha S, Choi H, Cheon GJ, Kang KW, Chung JK, Kim EE, et al. Autoclustering of non-small cell lung carcinoma subtypes on (18)F-FDG PET using texture analysis: a preliminary result. Nucl Med Mol Imaging. 2014;48:278–86. doi:10.1007/s13139-014-0283-3.
Chung C. Tyrosine kinase inhibitors for epidermal growth factor receptor gene mutation-positive non-small cell lung cancers: an update for recent advances in therapeutics. J Oncol Pharm Pract. 2015. doi:10.1177/1078155215577810.
Caicedo C, Garcia-Velloso MJ, Lozano MD, Labiano T, Vigil Diaz C, Lopez-Picazo JM, et al. Role of [(1)(8)F]FDG PET in prediction of KRAS and EGFR mutation status in patients with advanced non-small-cell lung cancer. Eur J Nucl Med Mol Imaging. 2014;41:2058–65. doi:10.1007/s00259-014-2833-4.
Ko KH, Hsu HH, Huang TW, Gao HW, Shen DH, Chang WC, et al. Value of (1)(8)F-FDG uptake on PET/CT and CEA level to predict epidermal growth factor receptor mutations in pulmonary adenocarcinoma. Eur J Nucl Med Mol Imaging. 2014;41:1889–97. doi:10.1007/s00259-014-2802-y.
Acknowledgments
Dr. Alison Johnson is thanked for proofreading of the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was waived for this type of study by the local ethics committee (Ref A12-D24-VOL13, Comité de protection des personnes Nord-Ouest III), since the PET scans were performed for clinical indications and the trial procedures were performed independent of usual clinical reporting.
Additional information
Mathieu Hatt and Nicolas Aide contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Fig. 1
Illustration of the in-house software used to define a 3-D box around the tumour, aiming at enclosing the tumour as well as excluding any nearby physiological or undesired uptake. For details see the Materials and Methods section. (GIF 466 kb)
Supplemental Fig. 2
Relationship between quantitative values extracted from PSF or PSF7 and OSEM images, assessed using Bland-Altman plots for SUVmax (a) and SUVmean (b) in tumour lesions. (GIF 34 kb)
Supplemental Fig. 3
Plots of TF features (CHAUC: area under the curve of the cumulative histogram; high-intensity larger area emphasis (HILAE); ZP: zone percentage) against tumours MATV for OSEM versus PSF reconstructions. (GIF 84 kb)
Supplemental Fig. 4
Plots of TF features (CHAUC: area under the curve of the cumulative histogram; high-intensity larger area emphasis (HILAE); ZP: zone percentage) against tumours MATV for OSEM versus PSF7 reconstructions. (GIF 72 kb)
Supplemental Fig. 5
Impact of the EARL harmonisation strategy on textural features using the FLAB algorithm independently on the three sets of reconstructions to delineate lesions. Textural features for the three reconstructions used. CHAUC: area under the curve of the cumulative histogram; high-intensity larger area emphasis (HILAE); ZP zone percentage. Data is shown as Tukey boxplots (lines displaying median, 25th and 75th percentiles; cross represents the mean value).*, **, and *** indicate two-tailed P < .05, P < .01, and P < .001, respectively. ns: non significant. Data represent the eight outliers (above the 90th percentile) observed with OSEM-PSF MATV and/or PSF7-PSF MATV when using a 50 % of SUVmax threshold. (GIF 39 kb)
Supplemental Fig. 6
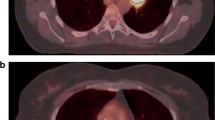
PET transverse slice of a PSF-reconstructed image showing one of the largest differences in MATV (−95 %) between FLAB and 50 % SUVmax thresholding. (GIF 22 kb)
Rights and permissions
About this article
Cite this article
Lasnon, C., Majdoub, M., Lavigne, B. et al. 18F-FDG PET/CT heterogeneity quantification through textural features in the era of harmonisation programs: a focus on lung cancer. Eur J Nucl Med Mol Imaging 43, 2324–2335 (2016). https://doi.org/10.1007/s00259-016-3441-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-016-3441-2