Abstract
Purpose
To study cerebral adenosine receptors (AR) in premanifest and manifest stages of Huntington’s disease (HD).
Methods
We quantified the cerebral binding potential (BP ND) of the A1AR in carriers of the HD CAG trinucleotide repeat expansion using the radioligand [18 F]CPFPX and PET. Four groups were investigated: (i) premanifest individuals far (preHD-A; n = 7) or (ii) near (preHD-B; n = 6) to the predicted symptom onset, (iii) manifest HD patients (n = 8), and (iv) controls (n = 36).
Results
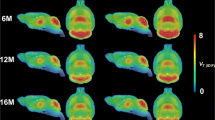
Cerebral A1AR values of preHD-A subjects were generally higher than those of controls (by up to 31 %, p < .01, in the thalamus on average). Across stages a successive reduction of A1AR BPND was observed to the levels of controls in preHD-B and undercutting controls in manifest HD by down to 25 %, p < .01, in the caudatus and amygdala. There was a strong correlation between A1AR BP ND and years to onset. Before onset of HD, the assumed annual rates of change of A1AR density were −1.2 % in the caudatus, −1.7 % in the thalamus and −3.4 % in the amygdala, while the corresponding volume losses amounted to 0.6 %, 0.1 % and 0.2 %, respectively.
Conclusions
Adenosine receptors switch from supra to subnormal levels during phenoconversion of HD. This differential regulation may play a role in the pathophysiology of altered energy metabolism.


Similar content being viewed by others
References
Langbehn DR, Hayden MR, Paulsen JS. CAG-repeat length and the age of onset in Huntington disease (HD): a review and validation study of statistical approaches. Am J Med Genet B Neuropsychiatr Genet. 2010;153B(2):397–408. doi:10.1002/ajmg.b.30992.
Kunig G, Leenders KL, Sanchez-Pernaute R, Antonini A, Vontobel P, Verhagen A, et al. Benzodiazepine receptor binding in Huntington′s disease: [11C]flumazenil uptake measured using positron emission tomography. Ann Neurol. 2000;47(5):644–8.
Feigin A, Tang C, Ma Y, Mattis P, Zgaljardic D, Guttman M, et al. Thalamic metabolism and symptom onset in preclinical Huntington’s disease. Brain. 2007;130(Pt 11):2858–67. doi:10.1093/brain/awm217.
Ciarmiello A, Giovacchini G, Orobello S, Bruselli L, Elifani F, Squitieri F. 18 F-FDG PET uptake in the pre-Huntington disease caudate affects the time-to-onset independently of CAG expansion size. Eur J Nucl Med Mol Imaging. 2012;39(6):1030–6. doi:10.1007/s00259-012-2114-z.
Tai YF, Pavese N, Gerhard A, Tabrizi SJ, Barker RA, Brooks DJ, et al. Microglial activation in presymptomatic Huntington’s disease gene carriers. Brain. 2007;130(Pt 7):1759–66. doi:10.1093/brain/awm044.
Ginovart N, Lundin A, Farde L, Halldin C, Backman L, Swahn CG, et al. PET study of the pre- and post-synaptic dopaminergic markers for the neurodegenerative process in Huntington’s disease. Brain. 1997;120(Pt 3):503–14.
Andrews TC, Weeks RA, Turjanski N, Gunn RN, Watkins LH, Sahakian B, et al. Huntington’s disease progression. PET and clinical observations. Brain. 1999;122(Pt 12):2353–63.
Pavese N, Andrews TC, Brooks DJ, Ho AK, Rosser AE, Barker RA, et al. Progressive striatal and cortical dopamine receptor dysfunction in Huntington’s disease: a PET study. Brain. 2003;126(Pt 5):1127–35.
Martin WR, Hayden MR. Cerebral glucose and dopa metabolism in movement disorders. Can J Neurol Sci. 1987;14(3 Suppl):448–51.
Bohnen NI, Koeppe RA, Meyer P, Ficaro E, Wernette K, Kilbourn MR, et al. Decreased striatal monoaminergic terminals in Huntington disease. Neurology. 2000;54(9):1753–9.
Bauer A, Holschbach MH, Meyer PT, Boy C, Herzog H, Olsson RA, et al. In vivo imaging of adenosine A1 receptors in the human brain with [18 F]CPFPX and positron emission tomography. Neuroimage. 2003;19(4):1760–9.
Holschbach MH, Olsson RA, Bier D, Wutz W, Sihver W, Schuller M, et al. Synthesis and evaluation of no-carrier-added 8-cyclopentyl-3-(3-[(18)F]fluoropropyl)-1-propylxanthine ([(18)F]CPFPX): a potent and selective A(1)-adenosine receptor antagonist for in vivo imaging. J Med Chem. 2002;45(23):5150–6.
Elmenhorst D, Meyer PT, Winz OH, Matusch A, Ermert J, Coenen HH, et al. Sleep deprivation increases A1 adenosine receptor binding in the human brain: a positron emission tomography study. J Neurosci. 2007;27(9):2410–5. doi:10.1523/JNEUROSCI.5066-06.2007.
Meyer PT, Elmenhorst D, Boy C, Winz O, Matusch A, Zilles K, et al. Effect of aging on cerebral A1 adenosine receptors: A [18 F]CPFPX PET study in humans. Neurobiol Aging. 2007;28(12):1914–24. doi:10.1016/j.neurobiolaging.2006.08.005.
Meyer PT, Elmenhorst D, Matusch A, Winz O, Zilles K, Bauer A. 18 F-CPFPX PET: on the generation of parametric images and the effect of scan duration. J Nucl Med. 2006;47(2):200–7.
Fredholm BB. Adenosine, an endogenous distress signal, modulates tissue damage and repair. Cell Death Differ. 2007;14(7):1315–23. doi:10.1038/sj.cdd.4402132.
Blum D, Hourez R, Galas MC, Popoli P, Schiffmann SN. Adenosine receptors and Huntington’s disease: implications for pathogenesis and therapeutics. Lancet Neurol. 2003;2(6):366–74.
Gianfriddo M, Melani A, Turchi D, Giovannini MG, Pedata F. Adenosine and glutamate extracellular concentrations and mitogen-activated protein kinases in the striatum of Huntington transgenic mice. Selective antagonism of adenosine A2A receptors reduces transmitter outflow. Neurobiol Dis. 2004;17(1):77–88.
Damiano M, Galvan L, Deglon N, Brouillet E. Mitochondria in Huntington’s disease. Biochim Biophys Acta. 2010;1802(1):52–61. doi:10.1016/j.bbadis.2009.07.012.
Bauer A, Zilles K, Matusch A, Holzmann C, Riess O, von Horsten S. Regional and subtype selective changes of neurotransmitter receptor density in a rat transgenic for the Huntington’s disease mutation. J Neurochem. 2005;94(3):639–50. doi:10.1111/j.1471-4159.2005.03169.x.
Penney Jr JB, Vonsattel JP, MacDonald ME, Gusella JF, Myers RH. CAG repeat number governs the development rate of pathology in Huntington’s disease. Ann Neurol. 1997;41(5):689–92. doi:10.1002/ana.410410521.
HuntigtonStudyGroup. Unified Huntington’s Disease Rating Scale: reliability and consistency. Mov Disord. 1996;11(2):136–42. doi:10.1002/mds.870110204.
Saft C, Andrich J, Meisel NM, Przuntek H, Muller T. Assessment of simple movements reflects impairment in Huntington’s disease. Mov Disord. 2006;21(8):1208–12. doi:10.1002/mds.20939.
Saft C, Schuttke A, Beste C, Andrich J, Heindel W, Pfleiderer B. fMRI reveals altered auditory processing in manifest and premanifest Huntington’s disease. Neuropsychologia. 2008;46(5):1279–89. doi:10.1016/j.neuropsychologia.2007.12.002.
Hurlemann R, Matusch A, Kuhn KU, Berning J, Elmenhorst D, Winz O, et al. 5-HT2A receptor density is decreased in the at-risk mental state. Psychopharmacology (Berl). 2008;195(4):579–90. doi:10.1007/s00213-007-0921-x.
van Oostrom JC, Maguire RP, Verschuuren-Bemelmans CC, van der Veenma Duin L, Pruim J, Roos RA, et al. Striatal dopamine D2 receptors, metabolism, and volume in preclinical Huntington disease. Neurology. 2005;65(6):941–3.
Tabrizi SJ, Reilmann R, Roos RA, Durr A, Leavitt B, Owen G, et al. Potential endpoints for clinical trials in premanifest and early Huntington’s disease in the TRACK-HD study: analysis of 24 month observational data. Lancet Neurol. 2012;11(1):42–53. doi:10.1016/S1474-4422(11)70263-0.
Kuhn A, Goldstein DR, Hodges A, Strand AD, Sengstag T, Kooperberg C, et al. Mutant huntingtin’s effects on striatal gene expression in mice recapitulate changes observed in human Huntington’s disease brain and do not differ with mutant huntingtin length or wild-type huntingtin dosage. Hum Mol Genet. 2007;16(15):1845–61.
Ren H, Stiles GL. A single-stranded DNA binding site in the human A1 adenosine receptor gene promoter. Mol Pharmacol. 1998;53(1):43–51.
Rivkees SA, Chen M, Kulkarni J, Browne J, Zhao Z. Characterization of the murine A1 adenosine receptor promoter, potent regulation by GATA-4 and Nkx2.5. J Biol Chem. 1999;274(20):14204–9.
Borovecki F, Lovrecic L, Zhou J, Jeong H, Then F, Rosas HD, et al. Genome-wide expression profiling of human blood reveals biomarkers for Huntington’s disease. Proc Natl Acad Sci U S A. 2005;102(31):11023–8. doi:10.1073/pnas.0504921102.
Eltzschig HK, Abdulla P, Hoffman E, Hamilton KE, Daniels D, Schonfeld C, et al. HIF-1-dependent repression of equilibrative nucleoside transporter (ENT) in hypoxia. J Exp Med. 2005;202(11):1493–505. doi:10.1084/jem.20050177.
Kennedy L, Shelbourne PF, Dewar D. Alterations in dopamine and benzodiazepine receptor binding precede overt neuronal pathology in mice modelling early Huntington disease pathogenesis. Brain Res. 2005;1039(1–2):14–21. doi:10.1016/j.brainres.2005.01.029.
Tarditi A, Camurri A, Varani K, Borea PA, Woodman B, Bates G, et al. Early and transient alteration of adenosine A2A receptor signaling in a mouse model of Huntington disease. Neurobiol Dis. 2006;23(1):44–53. doi:10.1016/j.nbd.2006.01.014.
Doolette DJ. Mechanism of adenosine accumulation in the hippocampal slice during energy deprivation. Neurochem Int. 1997;30(2):211–23.
Schindler M, Harris CA, Hayes B, Papotti M, Humphrey PP. Immunohistochemical localization of adenosine A1 receptors in human brain regions. Neurosci Lett. 2001;297(3):211–5.
Rivkees SA, Price SL, Zhou FC. Immunohistochemical detection of A1 adenosine receptors in rat brain with emphasis on localization in the hippocampal formation, cerebral cortex, cerebellum, and basal ganglia. Brain Res. 1995;677(2):193–203.
Johnston JB, Silva C, Gonzalez G, Holden J, Warren KG, Metz LM, et al. Diminished adenosine A1 receptor expression on macrophages in brain and blood of patients with multiple sclerosis. Ann Neurol. 2001;49(5):650–8.
Henley SM, Novak MJ, Frost C, King J, Tabrizi SJ, Warren JD. Emotion recognition in Huntington’s disease: a systematic review. Neurosci Biobehav Rev. 2012;36(1):237–53. doi:10.1016/j.neubiorev.2011.06.002.
Ille R, Holl AK, Kapfhammer HP, Reisinger K, Schafer A, Schienle A. Emotion recognition and experience in Huntington’s disease: is there a differential impairment? Psychiatry Res. 2011;188(3):377–82. doi:10.1016/j.psychres.2011.04.007.
Beste C, Saft C, Gunturkun O, Falkenstein M. Increased cognitive functioning in symptomatic Huntington’s disease as revealed by behavioral and event-related potential indices of auditory sensory memory and attention. J Neurosci. 2008;28(45):11695–702. doi:10.1523/JNEUROSCI.2659-08.2008.
Simonin C, Duru C, Salleron J, Hincker P, Charles P, Delval A, et al. Association between caffeine intake and age at onset in Huntington’s disease. Neurobiol Dis. 2013;58:179–82. doi:10.1016/j.nbd.2013.05.013.
Alfinito PD, Wang SP, Manzino L, Rijhsinghani S, Zeevalk GD, Sonsalla PK. Adenosinergic protection of dopaminergic and GABAergic neurons against mitochondrial inhibition through receptors located in the substantia nigra and striatum, respectively. J Neurosci. 2003;23(34):10982–7.
Huang NK, Lin JH, Lin JT, Lin CI, Liu EM, Lin CJ, et al. A new drug design targeting the adenosinergic system for Huntington’s disease. PLoS One. 2011;6(6):e20934. doi:10.1371/journal.pone.0020934.
Langbehn DR, Brinkman RR, Falush D, Paulsen JS, Hayden MR. A new model for prediction of the age of onset and penetrance for Huntington’s disease based on CAG length. Clin Genet. 2004;65(4):267–77. doi:10.1111/j.1399-0004.2004.
Saft C, Andrich J, Meisel NM, Przuntek H, Müller T. Assessment of complex movements reflects dysfunction in Huntington's disease. J Neurol. 2003;250(12):1469–74. doi:10.1007/s00415-003-0256-4.
Acknowledgements
We thank all patients and their families for their kind support. The authors gratefully acknowledge the fruitful discussions with J. Ermert, M. Holschbach, D. Bier and K. Hamacher as well as the excellent technical assistance of S. Grafmuller, M. Lang, B. Palm, S. Rehbein, E. Wabbals (Institute of Nuclear Chemistry), M. Vögeling (Molecular Neuroimaging Group), S. Schaden, L. Tellmann, E. Theelen, C. Frey (PET Instrumentation Group), B. Elghahwagi, P. Engels, G. Oefler, J. N. Shah (MRI Instrumentation Group, Research Center Juelich).
Sponsorship and funding
Expenses for the entire study and personal budget for A.M., D.E. and A.B.. were financed by Forschungszentrum Jülich. C.S. was supported by the Ruhr-University Bochum (FoRUM grant K040/09).
Author’s financial disclosures
For all authors, there was no sponsorship or funding related to the present study.
A. M., D.E. and A.B.. received no industrial funding.
R. G. has received payments for consultancy from Biogen and Teva. He has also received speaker honoraria and research grants from Biogen Idec Germany, Teva, Sanofi-Aventis, Novartis, Bayer Healthcare, and MerckSerono.
H. P. Hartung has received personal compensation for activities with Biogen Idec, Teva, Sanofi Aventis, Novartis Pharma, Merck Serono, and Bayer Schering.
P. H. K. received funding from Boehringer Ingelheim Pharma and TEVA.
C. S. received honorarium from Temmler Pharma GmbH & Co.KG for scientific talks, compensation in the context of the Registry-Study of the Euro-HD-Network, in the context of the ACR16-Study (Neurosearch), the AFQ-Study (Novartis), the Selisistat-Studies (Siena Biotech) and received research support for a research project with Teva Pharma GmbH and the Cure Huntington’s Disease Initiative (CHDI).
Author information
Authors and Affiliations
Corresponding author
Additional information
Individual contributions of the authors
Andreas Matusch: data analysis, statistics, interpretation, drafting and revising of the MS
Carsten Saft: conceptualization of the study, interpretation, drafting and revising of the MS
David Elmenhorst: data analysis, revising of the MS
Peter H. Kraus: revising the MS
Ralf Gold: revising the MS
Hans-Peter Hartung: revising the MS
Andreas Bauer: conceptualization of the study, interpretation of the data, revising the MS
Matusch and Saft are authors that contributed equally to this study.
Rights and permissions
About this article
Cite this article
Matusch, A., Saft, C., Elmenhorst, D. et al. Cross sectional PET study of cerebral adenosine A1 receptors in premanifest and manifest Huntington’s disease. Eur J Nucl Med Mol Imaging 41, 1210–1220 (2014). https://doi.org/10.1007/s00259-014-2724-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-014-2724-8