Abstract
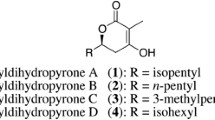
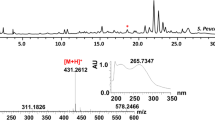
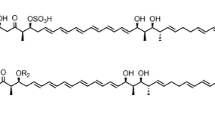
Spirolaxine is a natural product isolated from Sporotrichum laxum ATCC 15155, which has shown a variety of biological activities including promising anti-Helicobacter pylori property. To understand how this compound is biosynthesized, the genome of S. laxum was sequenced. Analysis of the genome sequence revealed two putative type III polyketide synthase (PKS) genes in this strain, Sl-pks1 and Sl-pks2, which are located adjacent to each other (~2.0 kb apart) in a tail-to-tail arrangement. Disruption of these two genes revealed that Sl-PKS2 is the dedicated PKS involved in the biosynthesis of spirolaxine. The intron-free Sl-pks2 gene was amplified from the cDNA of S. laxum and ligated into the expression vector pET28a for expression in Escherichia coli BL21-CodonPlus (DE3)-RIL. The major products of Sl-PKS2 in E. coli were characterized as alkylresorcinols that contain a C13–C17 saturated or unsaturated hydrocarbon side chain based on the spectral data. This enzyme was purified and reacted with malonyl-CoA and a series of fatty acyl-SNACs (C6–C10). Corresponding alkylresorcinols were formed from the decarboxylation of the synthesized tetraketide resorcylic acids, together with fatty acyl-primed triketide and tetraketide pyrones as byproducts. This work provides important information about the PKS involved in the biosynthesis of spirolaxine, which will facilitate further understanding and engineering of the biosynthetic pathway of this medicinally important molecule.





Similar content being viewed by others
References
Arnone A, Assante G, Nasini G, Vajna de Pava O (1990) Secondary mold metabolites. Part 28. Spirolaxine and sporotricale: two long-chain phthalides produced by Sporotrichum laxum. Phytochemistry 29:613–616
Baerson SR, Schröder J, Cook D, Rimando AM, Pan Z, Dayan FE, Noonan BP, Duke SO (2010) Alkylresorcinol biosynthesis in plants: new insights from an ancient enzyme family? Plant Signal Behav 5:1286–1289
Bava A, Clericuzio M, Giannini G, Malpezzi L, Meille SV, Nasini G (2005) Absolute configuration of the fungal metabolite spirolaxine. Eur J Org Chem 2005(11):2292–2296
Blaser MJ (1992) Helicobacter pylori: its role in disease. Clin Infect Dis 15(3):386–391
Dekker KA, Inagaki T, Gootz TD, Kaneda K, Nomura E, Sakakibara T, Sakemi S, Sugie Y, Yamauchi Y, Yoshikawa N, Kojima N (1997) CJ-12,954 and its congeners, new anti-Helicobacter pylori compounds produced by Phanerochaete velutina: fermentation, isolation, structural elucidation and biological activities. J Antibiot 50(10):833–839
Dimitrov I, Furkert DP, Fraser JD, Radcliff FJ, Finch O, Brimble MA (2012) Synthesis and anti-Helicobacter pylori activity of analogues of spirolaxine methyl ether. MedChemComm 3(8):938–943
Funa N, Awakawa T, Horinouchi S (2007) Pentaketide resorcylic acid synthesis by type III polyketide synthase from Neurospora crassa. J Biol Chem 282(19):14476–14481
Giannini G, Pisano C, Riccioni T, Marcellini M, Chiarucci I, Bava A, Nasini G (2004) Spirolaxine, a fungal metabolite from Sporotrichum laxum: determination of relative stereochemistry and biological activity in vitro. Proc Amer Assoc Cancer Res 45:1011
Hashimoto M, Koen T, Takahashi H, Suda C, Kitamoto K, Fujii I (2014) Aspergillus oryzae CsyB catalyzes the condensation of two beta-ketoacyl-CoAs to form 3-acetyl-4-hydroxy-6-alkyl-alpha-pyrone. J Biol Chem 289(29):19976–19984
Ishikawa J, Hotta K (1999) FramePlot: a new implementation of the frame analysis for predicting protein-coding regions in bacterial DNA with a high G + C content. FEMS Microbiol Lett 174(2):251–253
Jeya M, Kim TS, Tiwari MK, Li J, Zhao H, Lee JK (2012) The Botrytis cinerea type III polyketide synthase shows unprecedented high catalytic efficiency toward long chain acyl-CoAs. Mol BioSyst 8(11):2864–2867
Knodler M, Conrad J, Wenzig EM, Bauer R, Lacorn M, Beifuss U, Carle R, Schieber A (2008) Anti-inflammatory 5-(11’Z-heptadecenyl)- and 5-(8’Z,11’Z-heptadecadienyl)-resorcinols from mango (Mangifera indica L.) peels. Phytochemistry 69(4):988–993
Kozubek A, Tyman JH (1999) Resorcinolic lipids, the natural non-isoprenoid phenolic amphiphiles and their biological activity. Chem Rev 99(1):1–26
Li J, Luo Y, Lee JK, Zhao H (2011) Cloning and characterization of a type III polyketide synthase from Aspergillus niger. Bioorg Med Chem Lett 21(20):6085–6089
Mejía R, Gómez-Eichelmann MC, Fernández MS (1999) Fatty acid profile of Escherichia coli during the heat-shock response. IUBMB Life 47(5):835–844
Miyanaga A, Horinouchi S (2010) Type III polyketide synthases responsible for phenolic lipid synthesis. In: Timmis KN (ed) Handbook of hydrocarbon and lipid microbiology. Springer-Verlag, Berlin, Heidelberg, pp. 519–525
Robinson JE, Brimble MA (2007) Synthesis of the anti-Helicobacter pylori agent (+)-spirolaxine methyl ether and the unnatural (2″S)-diastereomer. Org Biomol Chem 5(16):2572–2582
Seshime Y, Juvvadi PR, Fujii I, Kitamoto K (2005) Discovery of a novel superfamily of type III polyketide synthases in Aspergillus oryzae. Biochem Biophys Res Commun 331(1):253–260
Shaw MK, Ingraham JL (1965) Fatty acid composition of Escherichia coli as a possible controlling factor of the minimal growth temperature. J Bacteriol 90(1):141–146
Stanke M, Morgenstern B (2005) AUGUSTUS: a web server for gene prediction in eukaryotes that allows user-defined constraints. Nucleic Acids Res 33(Web Server):W465-W467
Suzuki Y, Esumi Y, Uramoto M, Kono Y, Sakurai A (1997) Structural analyses of carbon chains in 5-alk(en)ylresorcinols of rye and wheat whole flour by tandem mass spectrometry. Biosci Biotechnol Biochem 61(3):480–486
Tsukamoto M, Sano H, Kadota Y, Ojiri K, Suda H (1998) Anticancer spirolaxine and its fermentative manufacture. JP10007557A,
Wang S, Zhang S, Zhou T, Zhan J (2013) Three new resorcylic acid derivatives from Sporotrichum laxum. Bioorg Med Chem Lett 23(21):5806–5809
White SW, Zheng J, Zhang Y-M, Rock CO (2005) The structural biology of type II fatty acid biosynthesis. Annu Rev Biochem 74:791–831
Xu Y, Zhou T, Espinosa-Artiles P, Tang Y, Zhan J, Molnar I (2014a) Insights into the biosynthesis of 12-membered resorcylic acid lactones from heterologous production in Saccharomyces cerevisiae. ACS Chem Biol 9(5):1119–1127
Xu Y, Zhou T, Zhang S, Espinosa-Artiles P, Wang L, Zhang W, Lin M, Gunatilaka AAL, Zhan J, Molnar I (2014b) Diversity-oriented combinatorial biosynthesis of benzenediol lactone scaffolds by subunit shuffling of fungal polyketide synthases. Proc Natl Acad Sci U S A 111(34):12354–12359
Xu Y, Zhou T, Zhang S, Xuan L-J, Zhan J, Molnar I (2013a) Thioesterase domains of fungal nonreducing polyketide synthases act as decision gates during combinatorial biosynthesis. J Am Chem Soc 135(29):10783–10791
Xu Y, Zhou T, Zhou Z, Su S, Roberts SA, Montfort WR, Zeng J, Chen M, Zhang W, Lin M, Zhan J, Molnar I (2013b) Rational reprogramming of fungal polyketide first-ring cyclization. Proc Natl Acad Sci U S A 110(14):5398–5403
Yu D, Zeng J, Chen D, Zhan J (2010) Characterization and reconstitution of a new fungal type III polyketide synthase from Aspergillus oryzae. Enzyme Microb Tech 46(7):575–580
Zhou H, Zhan J, Watanabe K, Xie X, Tang Y (2008) A polyketide macrolactone synthase from the filamentous fungus Gibberella zeae. Proc Natl Acad Sci U S A 105(17):6249–6254
Acknowledgments
This work was supported by a Grant-In-Aid from the American Heart Association (16GRNT26430067), grants from the National Natural Science Foundation of China (31170763, 31470787), a Hunan Science and Technology Key Project (2014FJ1007), and the Hundred Talents Program of Hunan Province, China.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare that they have no competing interests.
Electronic supplementary material
ESM 1
(PDF 473 kb)
Rights and permissions
About this article
Cite this article
Sun, L., Wang, S., Zhang, S. et al. Identification of a type III polyketide synthase involved in the biosynthesis of spirolaxine. Appl Microbiol Biotechnol 100, 7103–7113 (2016). https://doi.org/10.1007/s00253-016-7444-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-016-7444-5