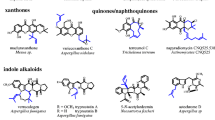
Abstract
Prenylated compounds are ubiquitously found in nature and demonstrate interesting biological and pharmacological activities. Prenyltransferases catalyze the attachment of prenyl moieties from different prenyl donors to various acceptors and contribute significantly to the structural and biological diversity of natural products. In the last decade, significant progress has been achieved for the prenyltransferases of the dimethylallyltryptophan synthase (DMATS) superfamily. More than 40 members of these soluble enzymes are identified in microorganisms and characterized biochemically. These enzymes were also successfully used for production of a large number of prenylated derivatives. N1-, C4-, C5-, C6-, and C7-prenylated tryptophan and N1-, C2-, C3-, C4-, and C7-prenylated tryptophan-containing peptides were obtained by using DMATS enzymes as biocatalysts. Tyrosine and xanthone prenyltransferases were used for production of prenylated derivatives of their analogs. More interestingly, the members of the DMATS superfamily demonstrated intriguing substrate and catalytic promiscuity and also used structurally quite different compounds as prenyl acceptors. Prenylated hydroxynaphthalenes, flavonoids, indolocarbazoles, and acylphloroglucinols, which are typical bacterial or plant metabolites, were produced by using several fungal DMATS enzymes. Furthermore, the potential usage of these enzymes was further expanded by using natural or unnatural DMAPP analogs as well as by coexpression with other genes like NRPS and by development of whole cell biocatalyst.






Similar content being viewed by others
References
Alcantara AR, Pace V, Hoyos P, Sandoval M, Holzer W, Hernaiz MJ (2014) Chemoenzymatic synthesis of carbohydrates as antidiabetic and anticancer drugs. Curr Top Med Chem 14:2694–2711
Botta B, Vitali A, Menendez P, Misiti D, Delle MG (2005) Prenylated flavonoids: pharmacology and biotechnology. Curr Med Chem 12:717–739
Botta B, Menendez P, Zappia G, de Lima RA, Torge R, Monachea GD (2009) Prenylated isoflavonoids: botanical distribution, structures, biological activities and biotechnological studies. An update (1995-2006). Curr Med Chem 16:3414–3468
Chen J, Morita H, Wakimoto T, Mori T, Noguchi H, Abe I (2012) Prenylation of a nonaromatic carbon of indolylbutenone by a fungal indole prenyltransferase. Org Lett 14:3080–3083
Chen R, Liu X, Zou J, Yin Y, Ou C, Li J, Wang R, Xie D, Zhang P, Dai J (2013) Regio- and stereospecific prenylation of flavonoids by Sophora flavescens prenyltransferases. Adv Synth Catal 355:1817–1828
Chooi YH, Cacho R, Tang Y (2010) Identification of the viridicatumtoxin and griseofulvin gene clusters from Penicillium aethiopicum. Chem Biol 17:483–494
Cui CB, Kakeya H, Okada G, Onose R, Ubukata M, Takahashi I, Isono K, Osada H (1995) Tryprostatins A and B, novel mammalian cell cycle inhibitors produced by Aspergillus fumigatus. J Antibiot 48:1382–1384
Edwards DJ, Gerwick WH (2004) Lyngbyatoxin biosynthesis: sequence of biosynthetic gene cluster and identification of a novel aromatic prenyltransferase. J Am Chem Soc 126:11432–11433
Fan A, Li S-M (2013) One substrate - seven products with different prenylation positions in one-step reactions: prenyltransferases make it possible. Adv Synth Catal 355:2659–2666
Fan A, Li S-M (2014) Prenylation of tyrosine and derivatives by a tryptophan C7-prenyltransferase. Tetrahedron Lett 55:5199–5202
Fan A, Chen H, Wu R, Xu H, Li S-M (2014) A new member of the DMATS superfamily from Aspergillus niger catalyzes prenylations of both tyrosine and tryptophan derivatives. Appl Microbiol Biotechnol 98:10119–10129
Fan A, Xie X, Li S-M (2015a) Tryptophan prenyltransferases showing higher catalytic activities for Friedel-Crafts alkylation of o- and m-tyrosines than tyrosine prenyltransferases. Org Biomol Chem 13:7551–7557
Fan A, Zocher G, Stec E, Stehle T, Li S-M (2015b) Site-directed mutagenesis switching a dimethylallyl tryptophan synthase to a specific tyrosine C3-prenylating enzyme. J Biol Chem 290:1364–1373
Gröger H, Hummel W (2014) Combining the 'two worlds' of chemocatalysis and biocatalysis towards multi-step one-pot processes in aqueous media. Curr Opin Chem Biol 19:171–179
Grundmann A, Li S-M (2005) Overproduction, purification and characterization of FtmPT1, a brevianamide F prenyltransferase from Aspergillus fumigatus. Microbiology 151:2199–2207
Haagen Y, Unsöld I, Westrich L, Gust B, Richard SB, Noel JP, Heide L (2007) A soluble, magnesium-independent prenyltransferase catalyzes reverse and regular C-prenylations and O-prenylations of aromatic substrates. FEBS Lett 581:2889–2893
Jost M, Zocher G, Tarcz S, Matuschek M, Xie X, Li S-M, Stehle T (2010) Structure-function analysis of an enzymatic prenyl transfer reaction identifies a reaction chamber with modifiable specificity. J Am Chem Soc 132:17849–17858
Kranen E, Steffan N, Mass R, Li S-M, Jose J (2011) Development of a whole cell biocatalyst for the efficient prenylation of indole derivatives by Autodisplay of the aromatic prenyltransferase FgaPT2. ChemCatChem 3:1200–1207
Kremer A, Li S-M (2008) Potential of a 7-dimethylallyltryptophan synthase as a tool for production of prenylated indole derivatives. Appl Microbiol Biotechnol 79:951–961
Kremer A, Westrich L, Li S-M (2007) A 7-dimethylallyltryptophan synthase from Aspergillus fumigatus: overproduction, purification and biochemical characterization. Microbiology 153:3409–3416
Kumano T, Richard SB, Noel JP, Nishiyama M, Kuzuyama T (2008) Chemoenzymatic syntheses of prenylated aromatic small molecules using Streptomyces prenyltransferases with relaxed substrate specificities. Bioorg Med Chem 16:8117–8126
Kumano T, Tomita T, Nishiyama M, Kuzuyama T (2010) Functional characterization of the promiscuous prenyltransferase responsible for furaquinocin biosynthesis: identification of a physiological polyketide substrate and its prenylated reaction products. J Biol Chem 285:39663–39671
Kuzuyama T, Noel JP, Richard SB (2005) Structural basis for the promiscuous biosynthetic prenylation of aromatic natural products. Nature 435:983–987
Li S-M (2009a) Applications of dimethylallyltryptophan synthases and other indole prenyltransferases for structural modification of natural products. Appl Microbiol Biotechnol 84:631–639
Li S-M (2009b) Evolution of aromatic prenyltransferases in the biosynthesis of indole derivatives. Phytochemistry 70:1746–1757
Li S-M (2010) Prenylated indole derivatives from fungi: structure diversity, biological activities, biosynthesis and chemoenzymatic synthesis. Nat Prod Rep 27:57–78
Li H, Ban Z, Qin H, Ma L, King AJ, Wang G (2015) A heteromeric membrane-bound prenyltransferase complex from hop catalyzes three sequential aromatic prenylations in the bitter acid pathway. Plant Physiol 167:650–659
Liebhold M, Li S-M (2013) Regiospecific benzylation of tryptophan and derivatives catalyzed by a fungal dimethylallyl transferase. Org Lett 15:5834–5837
Liebhold M, Xie X, Li S-M (2012) Expansion of enzymatic Friedel-Crafts alkylation on indoles: Acceptance of unnatural beta-unsaturated allyl diphospates by dimethylallyl-tryptophan synthases. Org Lett 14:4884–4885
Liebhold M, Xie X, Li S-M (2013) Breaking cyclic dipeptide prenyltransferase regioselectivity by unnatural alkyl donors. Org Lett 15:3062–3065
Liu AH, Liu DQ, Liang TJ, Yu XQ, Feng MT, Yao LG, Fang Y, Wang B, Feng LH, Zhang MX, Mao SC (2013) Caulerprenylols A and B, two rare antifungal prenylated para-xylenes from the green alga Caulerpa racemosa. Bioorg Med Chem Lett 23:2491–2494
Luk LYP, Tanner ME (2009) Mechanism of dimethylallyltryptophan synthase: evidence for a dimethylallyl cation intermediate in an aromatic prenyltransferase reaction. J Am Chem Soc 131:13932–13933
Luk LY, Qian Q, Tanner ME (2011) A cope rearrangement in the reaction catalyzed by dimethylallyltryptophan synthase? J Am Chem Soc 133:12342–12345
Maiya S, Grundmann A, Li S-M, Turner G (2006) The fumitremorgin gene cluster of Aspergillus fumigatus: identification of a gene encoding brevianamide F synthetase. Chembiochem 7:1062–1069
Maiya S, Grundmann A, Li S-M, Turner G (2009) Improved tryprostatin B production by heterologous gene expression in Aspergillus nidulans. Fungal Genet Biol 46:436–440
Metzger U, Schall C, Zocher G, Unsöld I, Stec E, Li S-M, Heide L, Stehle T (2009) The structure of dimethylallyl tryptophan synthase reveals a common architecture of aromatic prenyltransferases in fungi and bacteria. Proc Natl Acad Sci U S A 106:14309–14314
Mundt K, Li S-M (2013) CdpC2PT, a reverse prenyltransferase from Neosartorya fischeri with distinct substrate preference from known C2-prenyltransferases. Microbiology 159:2169–2179
Okamoto R, Izumi M, Kajihara Y (2014) Decoration of proteins with sugar chains: recent advances in glycoprotein synthesis. Curr Opin Chem Biol 22:92–99
Oya A, Tanaka N, Kusama T, Kim SY, Hayashi S, Kojoma M, Hishida A, Kawahara N, Sakai K, Gonoi T, Kobayashi J (2015) Prenylated benzophenones from Triadenum japonicum. J Nat Prod 78:258–264
Pockrandt D, Li S-M (2013) Geranylation of cyclic dipeptides by the dimethylallyl transferase AnaPT resulting in a shift of prenylation position on the indole ring. Chembiochem 14:2023–2028
Pockrandt D, Ludwig L, Fan A, König GM, Li S-M (2012) New insights into the biosynthesis of prenylated xanthones: XptB from Aspergillus nidulans catalyses an O-prenylation of xanthones. Chembiochem 13:2764–2771
Pockrandt D, Sack C, Kosiol T, Li S-M (2014) A promiscuous prenyltransferase from Aspergillus oryzae catalyses C-prenylations of hydroxynaphthalenes in the presence of different prenyl donors. Appl Microbiol Biotechnol 98:4987–4994
Raju R, Piggott AM, Huang XC, Capon RJ (2011) Nocardioazines: a novel bridged diketopiperazine scaffold from a marine-derived bacterium inhibits p-glycoprotein. Org Lett 13:2770–2773
Ruan H-L, Stec E, Li S-M (2009) Production of diprenylated indole derivatives by tandem incubation of two recombinant dimethylallyltryptophan synthases. Arch Microbiol 191:791–795
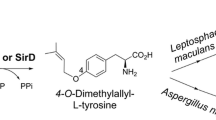
Rudolf JD, Poulter CD (2013) Tyrosine O-prenyltransferase SirD catalyzes S-, C-, and N-prenylations on tyrosine and tryptophan derivatives. ACS Chem Biol 8:2707–2714
Rudolf JD, Wang H, Poulter CD (2013) Multisite prenylation of 4-substituted tryptophans by dimethylallyltryptophan synthase. J Am Chem Soc 135:1895–1902
Salas JA, Mendez C (2009) Indolocarbazole antitumour compounds by combinatorial biosynthesis. Curr Opin Chem Biol 13:152–160
Sánchez C, Méndez C, Salas JA (2006) Indolocarbazole natural products: occurrence, biosynthesis, and biological activity. Nat Prod Rep 23:1007–1045
Sasaki K, Tsurumaru Y, Yamamoto H, Yazaki K (2011) Molecular characterization of a membrane-bound prenyltransferase specific for isoflavone from Sophora flavescens. J Biol Chem 286:24125–24134
Schkeryantz JM, Woo JCG, Siliphaivanh P, Depew KM, Danishefsky SJ (1999) Total synthesis of gypsetin, deoxybrevianamide E, brevianamide E, and tryprostatin B: novel constructions of 2,3-disubstituted indoles. J Am Chem Soc 121:11964–11975
Schuller JM, Zocher G, Liebhold M, Xie X, Stahl M, Li S-M, Stehle T (2012) Structure and catalytic mechanism of a cyclic dipeptide prenyltransferase with broad substrate promiscuity. J Mol Biol 422:87–99
Schultz AW, Lewis CA, Luzung MR, Baran PS, Moore BS (2010) Functional characterization of the cyclomarin/cyclomarazine prenyltransferase CymD directs the biosynthesis of unnatural cyclic peptides. J Nat Prod 73:373–377
Schwarzer DD, Gritsch PJ, Gaich T (2012) Mimicking dimethylallyltryptophan synthase: experimental evidence for a biosynthetic cope rearrangement process. Angew Chem Int Ed Engl 51:11514–11516
Schwarzer DD, Gritsch PJ, Gaich T (2013) How to 'cope' with the prenylation of the indole C4 position. Synlett 24:1025–1031
Steffan N, Li S-M (2009) Increasing structure diversity of prenylated diketopiperazine derivatives by using a 4-dimethylallyltryptophan synthase. Arch Microbiol 191:461–466
Steffan N, Unsöld IA, Li S-M (2007) Chemoenzymatic synthesis of prenylated indole derivatives by using a 4-dimethylallyltryptophan synthase from Aspergillus fumigatus. Chembiochem 8:1298–1307
Steffan N, Grundmann A, Yin W-B, Kremer A, Li S-M (2009) Indole prenyltransferases from fungi: a new enzyme group with high potential for the production of prenylated indole derivatives. Curr Med Chem 16:218–231
Subramanian S, Shen X, Yuan Q, Yan Y (2012) Identification and biochemical characterization of a 5-dimethylallyltryptophan synthase in Streptomyces coelicolor A3(2). Process Biochem 47:1419–1422
Sunassee SN, Davies-Coleman MT (2012) Cytotoxic and antioxidant marine prenylated quinones and hydroquinones. Nat Prod Rep 29:513–535
Tarcz S, Ludwig L, Li S-M (2014a) AstPT catalyses both reverse N1- and regular C2-prenylation of a methylated bisindolyl benzoquinone. Chembiochem 15:108–116
Tarcz S, Xie X, Li S-M (2014b) Substrate and catalytic promiscuity of secondary metabolite enzymes: O-prenylation of hydroxyxanthones with different prenyl donors by a bisindolyl benzoquinone C- and N-prenyltransferase. RSC Adv 4:17986–17992
Thandavamurthy K, Sharma D, Porwal SK, Ray D, Viswanathan R (2014) Regioselective Cope rearrangement and prenyl transfers on indole scaffold mimicking fungal and bacterial dimethylallyltryptophan synthases. J Org Chem 79:10049–10067
Tsurumaru Y, Sasaki K, Miyawaki T, Uto Y, Momma T, Umemoto N, Momose M, Yazaki K (2012) HlPT-1, a membrane-bound prenyltransferase responsible for the biosynthesis of bitter acids in hops. Biochem Biophys Res Commun 417:393–398
Unsöld IA, Li S-M (2005) Overproduction, purification and characterization of FgaPT2, a dimethylallyltryptophan synthase from Aspergillus fumigatus. Microbiology 151:1499–1505
Winkelblech J, Li S-M (2014) Biochemical investigations of two 6-DMATS enzymes from Streptomyces revealing novel features of L-tryptophan prenyltransferases. Chembiochem 15:1030–1039
Winkelblech J, Fan A, Li S-M (2015a) Prenyltransferases as key enzymes in the biosynthesis of prenylated natural products. Appl Microbiol Biotechnol. doi:10.1007/s00253-015-6811-y
Winkelblech J, Liebhold M, Gunera J, Xie X, Kolb P, Li S-M (2015b) Tryptophan C5-, C6- and C7-prenylating enzymes displaying a preference for C-6 of the indole ring in the presence of unnatural dimethylallyl diphosphate analogues. Adv Synth Catal 357:975–986
Wollinsky B, Ludwig L, Xie X, Li S-M (2012) Breaking the regioselectivity of indole prenyltransferases: identification of regular C3-prenylated hexahydropyrrolo[2,3-b]indoles as side products of the regular C2-prenyltransferase FtmPT1. Org Biomol Chem 10:9262–9270
Wunsch C, Mundt K, Li S-M (2015a) Targeted production of secondary metabolites by coexpression of non-ribosomal peptide synthetase and prenyltransferase genes in Aspergillus. Appl Microbiol Biotechnol 99:4213–4223
Wunsch C, Zou HX, Linne U, Li S-M (2015b) C7-prenylation of tryptophanyl and O-prenylation of tyrosyl residues in dipeptides by an Aspergillus terreus prenyltransferase. Appl Microbiol Biotechnol 99:1719–1730
Yamakawa T, Ideue E, Shimokawa J, Fukuyama T (2010) Total synthesis of tryprostatins A and B. Angew Chem Int Ed Engl 49:9262–9265
Yin W-B, Cheng J, Li S-M (2009a) Stereospecific synthesis of aszonalenins by using two recombinant prenyltransferases. Org Biomol Chem 7:2202–2207
Yin W-B, Grundmann A, Cheng J, Li S-M (2009b) Acetylaszonalenin biosynthesis in Neosartorya fischeri: Identification of the biosynthetic gene cluster by genomic mining and functional proof of the genes by biochemical investigation. J Biol Chem 284:100–109
Yin W-B, Xie X-L, Matuschek M, Li S-M (2010a) Reconstruction of pyrrolo[2,3-b]indoles carrying an α-configured reverse C3-dimethylallyl moiety by using recombinant enzymes. Org Biomol Chem 8:1133–1141
Yin W-B, Yu X, Xie X-L, Li S-M (2010b) Preparation of pyrrolo[2,3-b]indoles carrying a ß-configured reverse C3-dimethylallyl moiety by using a recombinant prenyltransferase CdpC3PT. Org Biomol Chem 8:2430–2438
Yin S, Yu X, Wang Q, Liu XQ, Li S-M (2013) Identification of a brevianamide F reverse prenyltransferase BrePT from Aspergillus versicolor with a broad substrate specificity towards tryptophan-containing cyclic dipeptides. Appl Microbiol Biotechnol 97:1649–1660
Yu X, Li S-M (2011) Prenylation of flavonoids by using a dimethylallyltryptophan synthase 7-DMATS from Aspergillus fumigatus. Chembiochem 12:2280–2283
Yu X, Li S-M (2012) Prenyltransferases of the dimethylallyltryptophan synthase superfamily. Methods Enzymol 516:259–278
Yu X, Xie X, Li S-M (2011) Substrate promiscuity of secondary metabolite enzymes: prenylation of hydroxynaphthalenes by fungal indole prenyltransferases. Appl Microbiol Biotechnol 92:737–748
Yu X, Yang A, Lin W, Li S-M (2012a) Friedel-Crafts alkylation on indolocarbazoles catalyzed by two dimethylallyltryptophan synthases from Aspergillus. Tetrahedron Lett 53:6861–6864
Yu X, Liu Y, Xie X, Zheng X-D, Li S-M (2012b) Biochemical characterization of indole prenyltransferases: Filling the last gap of prenylation positions by a 5-dimethylallyltryptophan synthase from Aspergillus clavatus. J Biol Chem 287:1371–1380
Yu X, Zocher G, Xie X, Liebhold M, Schütz S, Stehle T, Li S-M (2013) Catalytic mechanism of stereospecific formation of cis-configured prenylated pyrroloindoline diketopiperazines by indole prenyltransferases. Chem Biol 20:1492–1501
Yu H, Liebhold M, Xie X, Li S-M (2015) Tyrosine O-prenyltransferases TyrPT and SirD displaying similar behavior toward unnatural alkyl or benzyl diphosphate as their natural prenyl donor dimethylallyl diphosphate. Appl Microbiol Biotechnol:DOI 10.1007/s00253-015-6452-1
Zhao S, Smith KS, Deveau AM, Dieckhaus CM, Johnson MA, Macdonald TL, Cook JM (2002) Biological activity of the tryprostatins and their diastereomers on human carcinoma cell lines. J Med Chem 45:1559–1562
Zhao L, May JP, Huang J, Perrin DM (2012) Stereoselective synthesis of brevianamide E. Org Lett 14:90–93
Zhou K, Ludwig L, Li S-M (2015) Friedel-Crafts alkylation of acylphloroglucinols catalyzed by a fungal indole prenyltransferase. J Nat Prod 78:929–933
Zou H, Zheng X, Li S-M (2009) Substrate promiscuity of the cyclic dipeptide prenyltransferases from Aspergillus fumigatus. J Nat Prod 72:44–52
Zou H-X, Xie X-L, Linne U, Zheng X-D, Li S-M (2010) Simultaneous C7- and N1-prenylation of cyclo-L-Trp-L-Trp catalyzed by a prenyltransferase from Aspergillus oryzae. Org Biomol Chem 8:3037–3044
Zou H-X, Xie X, Zheng X-D, Li S-M (2011) The tyrosine O-prenyltransferase SirD catalyzes O-, N-, and C-prenylations. Appl Microbiol Biotechnol 89:1443–1451
Acknowledgments
The works in the author’s laboratory were supported in part by a grant from Deutsche Forschungsgemeinschaft (Li844/4-1 to S.-M. Li.). Aili Fan is a recipient of a scholarship from China scholarship council. Julia Winkelblech is partially financed by the LOEWE program of the State of Hessen (SynMikro to S.-M. Li).
Conflict of interest
The authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fan, A., Winkelblech, J. & Li, SM. Impacts and perspectives of prenyltransferases of the DMATS superfamily for use in biotechnology. Appl Microbiol Biotechnol 99, 7399–7415 (2015). https://doi.org/10.1007/s00253-015-6813-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-015-6813-9