Abstract
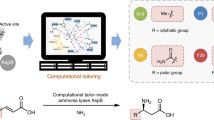
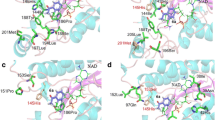
The present work created an esterase variant from Rhodobacter sphaeroides (RspE) with enhanced selectivity in hydrolytic kinetic resolutions by directed evolution. A “model” substrate, methyl mandelate, was introduced in the high-throughput screening procedure. E values of a variant CH (Asn62Cys/Leu145His) for six different esters were 10–83, which were a relative improvement compared to 2–20 for the wild type. Our subsequent crystal structure interpretation and molecular dynamics simulations helped shed light on the source of enantioselectivity modified by directed evolution. Though mutations displayed no “direct” interaction with the substrate, they were hypothesized to strengthen the intramolecular interaction in the catalytic cavity of variant. Conformation analysis revealed that the enhanced enantioselectivity of variant CH for the seven substrates applied in this study was derived from the decrease in size of the substrate binding pocket.





Similar content being viewed by others
References
Adams PD, Grosse-Kunstleve RW, Hung LW, Ioerger TR, McCoy AJ, Moriarty NW, Read RJ, Sacchettini JC, Sauter NK, Terwilliger TC (2002) PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr D: Biol Crystallogr 58:1948–1954
Arnold FH (2001) Combinatorial and computational challenges for biocatalyst design. Nature 409(6817):253–257. doi:10.1038/35051731
Bartsch S, Kourist R, Bornscheuer UT (2008) Complete inversion of enantioselectivity towards acetylated tertiary alcohols by a double mutant of a Bacillus subtilis esterase. Angew Chem Int Ed 47(8):1508–1511
Baumann M, Hauer BH, Bornscheuer UT (2000) Rapid screening of hydrolases for the enantioselective conversion of “difficult-to-resolve” substrates. Tetrahedron: Asymmetry 11(23):4781–4790
Beck G (2002) Synthesis of chiral drug substances. Synlett 6:837–850
Berendsen HJC, Postma JPM, Vangunsteren WF, Dinola A, Haak JR (1984) Molecular-dynamics with coupling to an external bath. J Chem Phys 81(8):3684–3690
Bloom JD, Arnold FH (2009) In the light of directed evolution: pathways of adaptive protein evolution. Proc Natl Acad Sci U S A 106:9995–10000
Brunger AT (2007) Version 1.2 of the crystallography and NMR system. Nat Protoc 2(11):2728–2733. doi:10.1038/nprot.2007.406
Brustad EM, Arnold FH (2011) Optimizing non-natural protein function with directed evolution. Curr Opin Chem Biol 15(2):201–210
Emsley P, Cowtan K (2004) Coot: model-building tools for molecular graphics. Acta Crystallogr D: Biol Crystallogr 60:2126–2132. doi:10.1107/s0907444904019158
Engstrom K, Nyhlen J, Sandstrom AG, Backvall JE (2010) Directed evolution of an enantioselective lipase with broad substrate scope for hydrolysis of alpha-substituted esters. J Am Chem Soc 132(20):7038–7042. doi:10.1021/Ja100593j
Gu JL, Liu J, Yu HW (2011) Quantitative prediction of enantioselectivity of Candida antarctica lipase B by combining docking simulations and quantitative structure–activity relationship (QSAR) analysis. J Mol Catal B Enzym 72(3–4):238–247. doi:10.1016/j.molcatb.2011.06.011
Guex N, Peitsch MC (1997) SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis 18(15):2714–2723
Hess B, Bekker H, Berendsen HJC, Fraaije JGEM (1997) LINCS: a linear constraint solver for molecular simulations. J Comput Chem 18(12):1463–1472
Ivancic M, Valinger G, Gruber K, Schwab H (2007) Inverting enantioselectivity of Burkholderia gladioli esterase EstB by directed and designed evolution. J Biotechnol 129(1):109–122
Langer G, Cohen SX, Lamzin VS, Perrakis A (2008) Automated macromolecular model building for X-ray crystallography using ARP/wARP version 7. Nat Protoc 3(7):1171–1179. doi:10.1038/nprot.2008.91
Laskowski RA, Macarthur MW, Moss DS, Thornton JM (1993) Procheck—a program to check the stereochemical quality of protein structures. J Appl Crystallogr 26:283–291
Liu J, Tang XL, Wang B, Yu HW, Min H (2010) Cloning, screening and characterization of ester hydrolases with enantioselectivity in typical bacteria. Process Biochem 45(4):475–480. doi:10.1016/j.procbio.2009.11.004
Murshudov GN, Vagin AA, Dodson EJ (1997) Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr D: Biol Crystallogr 53:240–255
Nyhlen J, Martin-Matute B, Sandstrom AG, Bocola M, Backvall JE (2008) Influence of delta-functional groups on the enantiorecognition of secondary alcohols by Candida antarctica lipase B. ChemBioChem 9(12):1968–1974. doi:10.1002/cbic.200800036
Päiviö M, Mavrynsky D, Leino R, Kanerva LT (2011) Dynamic kinetic resolution of a wide range of secondary alcohols: cooperation of dicarbonylchlorido (pentabenzylcyclopentadienyl) ruthenium and CAL-B. Eur J Org Chem 2011(8):1452–1457
Perrakis A, Harkiolaki M, Wilson KS, Lamzin VS (2001) ARP/wARP and molecular replacement. Acta Crystallogr D: Biol Crystallogr 57:1445–1450
Petrounia IP, Arnold FH (2000) Designed evolution of enzymatic properties. Curr Opin Biotechnol 11(4):325–330
Puschett JB, Rao BS, Karandikar BM, Matyjaszewski K (1991) Indicator characteristics of bromothymol blue derivatives. Talanta 38(3):335–338
Reetz MT (2004) Changing the enantioselectivity of enzymes by directed evolution. Protein Eng 388:238–256
Reetz MT, Puls M, Carballeira JD, Vogel A, Jaeger KE, Eggert T, Thiel W, Bocola M, Otte N (2007) Learning from directed evolution: further lessons from theoretical investigations into cooperative mutations in lipase enantioselectivity. ChemBioChem 8(1):106–112. doi:10.1002/cbic.200600359
Reetz MT, Bocola M, Wang LW, Sanchis J, Cronin A, Arand M, Zou J, Archelas A, Bottalla AL, Naworyta A, Mowbray SL (2009) Directed evolution of an enantioselective epoxide hydrolase: uncovering the source of enantioselectivity at each evolutionary stage. J Am Chem Soc 131(21):7334–7343. doi:10.1021/ja809673d
Schmid A, Dordick JS, Hauer B, Kiener A, Wubbolts M, Witholt B (2001) Industrial biocatalysis today and tomorrow. Nature 409(6817):258–268
Schneider TR, Sheldrick GM (2002) Substructure solution with SHELXD. Acta Crystallogr D: Biol Crystallogr 58:1772–1779. doi:10.1107/s0907444902011678
Sheldrick GM (2008) A short history of SHELX. Acta Crystallogr A 64:112–122. doi:10.1107/s0108767307043930
Sheldrick GM (2010) Experimental phasing with SHELXC/D/E: combining chain tracing with density modification. Acta Crystallogr D: Biol Crystallogr 66:479–485. doi:10.1107/s0907444909038360
Torshin IY, Weber IT, Harrison RW (2002) Geometric criteria of hydrogen bonds in proteins and identification of ‘bifurcated’ hydrogen bonds. Protein Eng 15(5):359–363
Van der Spoel D, Lindahl E, Hess B, Groenhof G, Mark AE, Berendsen HJC (2005) GROMACS: fast, flexible, and free. J Comput Chem 26(16):1701–1718. doi:10.1002/Jcc.20291
Wang B, Tang XL, Ren GF, Liu J, Yu HW (2009) A new high-throughput screening method for determining active and enantioselective hydrolases. Biochem Eng J 46(3):345–349. doi:10.1016/j.bej.2009.06.002
Whitesell JK, Reynolds D (1983) Resolution of chiral alcohols with mandelic-acid. J Org Chem 48(20):3548–3551
Wong TS, Arnold FH, Schwaneberg U (2004) Laboratory evolution of cytochrome P450BM-3 monooxygenase for organic cosolvents. Biotechnol Bioeng 85(3):351–358. doi:10.1002/Bit.10896
Zingg SP, Arnett EM, Mcphail AT, Bothnerby AA, Gilkerson WR (1988) Chiral discrimination in the structures and energetics of association of stereoisomeric salts of mandelic-acid with alpha-phenethylamine, ephedrine, and pseudoephedrine. J Am Chem Soc 110(5):1565–1580
Acknowledgment
This work was financially supported by the Natural Science Foundation of China (grant no. 21176215/21176102), Outstanding Young Scholar of Zhejiang Province (grant no. R4110092) and the Program for Zhejiang Leading Team of S&T Innovation (2011R50007). We are grateful for all editor and reviewers for their helpful advice. We also thank all the other members of Prof. Yu’s group.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Ma Jingbo and Wu Lian contributed equally.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 370 kb)
Rights and permissions
About this article
Cite this article
Ma, J., Wu, L., Guo, F. et al. Enhanced enantioselectivity of a carboxyl esterase from Rhodobacter sphaeroides by directed evolution. Appl Microbiol Biotechnol 97, 4897–4906 (2013). https://doi.org/10.1007/s00253-012-4396-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-012-4396-2