Abstract
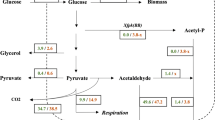
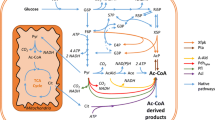
Several bacterial species and filamentous fungi utilize the phosphoketolase pathway (PHK) for glucose dissimilation as an alternative to the Embden–Meyerhof–Parnas pathway. In Aspergillus nidulans, the utilization of this metabolic pathway leads to increased carbon flow towards acetate and acetyl CoA. In the first step of the PHK, the pentose phosphate pathway intermediate xylulose-5-phosphate is converted into acetylphosphate and glyceraldehyde-3-phosphate through the action of xylulose-5-phosphate phosphoketolase, and successively acetylphosphate is converted into acetate by the action of acetate kinase. In the present work, we describe a metabolic engineering strategy used to express the fungal genes of the phosphoketolase pathway in Saccharomyces cerevisiae and the effects of the expression of this recombinant route in yeast. The phenotype of the engineered yeast strain MP003 was studied during batch and chemostat cultivations, showing a reduced biomass yield and an increased acetate yield during batch cultures. To establish whether the observed effects in the recombinant strain MP003 were due directly or indirectly to the expression of the phosphoketolase pathway, we resolved the intracellular flux distribution based on 13C labeling during chemostat cultivations. From flux analysis it is possible to conclude that yeast is able to use the recombinant pathway. Our work indicates that the utilization of the phosphoketolase pathway does not interfere with glucose assimilation through the Embden–Meyerhof–Parnas pathway and that the expression of this route can contribute to increase the acetyl CoA supply, therefore holding potential for future metabolic engineering strategies having acetyl CoA as precursor for the biosynthesis of industrially relevant compounds.




Similar content being viewed by others
References
Christensen B, Gombert AK, Nielsen J (2002) Analysis of flux estimates based on (13)C-labelling experiments. Eur J Biochem 269:2795–800
Chung AE (1970) Pyridine nucleotide transhydrogenase from Azotobacter vinelandii. J Bacteriol 2:438–47
Ciriacy M, Breitenbach I (1979) Physiological effects of seven different blocks in glycolysis in Saccharomyces cerevisiae. J Bacteriol 1:152–160
Compagno C, Boschi F, Ranzi BM (1996) Glycerol production in a triose phosphate isomerase deficient mutant of Saccharomyces cerevisiae. Biotechnol Prog 12:591–595
Dauner M, Sauer U (2000) GC-MS analysis of amino acids rapidly provides rich information for isotopomer balancing. Biotechnol Prog 16:642–9
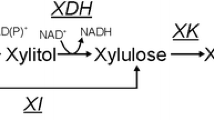
Evans T, Ratledge C (1984) Induction of xylulose-5-phosphate phosphoketolase in a variety of yeasts grown on d-xylose: the key to efficient xylose metabolism. Arch Microbiol 139:48–52
Fleige C, Kroll J, Steinbuchel A (2011) Establishment of an alternative phosphoketolase-dependent pathway for fructose catabolism in Ralstonia eutropha H16. Appl Microbiol Biotechnol 91:769–776
Gombert AK, Moreira dos Santos M, Christensen B, Nielsen J (2001) Network identification and flux quantification in the central metabolism of Saccharomyces cerevisiae under different conditions of glucose repression. J Bacteriol 183:1441–51
Gombert AK, Nielsen J (2000) Mathematical modelling of metabolism. Curr Opin Biotechnol 11:180–6
Grotkjaer T, Christakopoulos P, Nielsen J, Olsson L (2005) Comparative metabolic network analysis of two xylose fermenting recombinant Saccharomyces cerevisiae strains. Metab Eng 7:437–44
Ingram-Smith C, Martin SR, Smith KS (2006) Acetate kinase: not just a bacterial enzyme. Trends Microbiol 14:249–53
Kawai S, Hashimoto W, Murata K (2010) Transformation of Saccharomyces cerevisiae and other fungi: methods and possible underlying mechanism. Bioeng Bugs 1:395–403
Lam KB, Marmur J (1979) Isolation and characterization of Saccharomyces cerevisiae glycolytic pathway mutants. J Bacteriol 2:746–9
Marx A, de Graaf AA, Wiechert W, Eggeling L, Sahm H (1996) Determination of the fluxes in the central metabolism of Corynebacterium glutamicum by nuclear magnetic resonance spectroscopy combined with metabolite balancing. Biotechnol Bioeng 49:111–29
Meile L, Rohr LM, Geissmann TA, Herensperger M, Teuber M (2001) Characterization of the d-xylulose 5-phosphate/d-fructose 6-phosphate phosphoketolase gene (xfp) from Bifidobacterium lactis. J Bacteriol 183:2929–36
Nissen TL, Anderlund M, Nielsen J, Villadsen J, Kielland-Brandt MC (2001) Expression of a cytoplasmic transhydrogenase in Saccharomyces cerevisiae results in formation of 2-oxoglutarate due to depletion of the NADPH pool. Yeast 18:19–32
Panagiotou G, Andersen MR, Grotkjaer T, Regueira TB, Hofmann G, Nielsen J, Olsson L (2008) Systems Analysis Unfolds the Relationship between the Phosphoketolase Pathway and Growth in Aspergillus nidulans. PLoS One 12:e3847
Papini M, Nookaew I, Scalcinati G, Siewers V, Nielsen J (2010) Phosphoglycerate mutase knock-out mutant Saccharomyces cerevisiae: physiological investigation and transcriptome analysis. Biotechnol J 5:1016–1027
Partow S, Siewers V, Bjørn S, Nielsen J, Maury J (2010) Characterization of different promoters for designing a new expression vector in Saccharomyces cerevisiae. Yeast 27:955–64
Petersen S, Mack C, de Graaf AA, Riedel C, Eikmanns BJ, Sahm H (2001) Metabolic consequences of altered phosphoenolpyruvate carboxykinase activity in Corynebacterium glutamicum reveal anaplerotic regulation mechanisms in vivo. Metab Eng 3:344–61
Presecan-Siedel E, Galinier A, Longin R, Deutscher J, Danchin A, Glaser P, Martin-Verstraete I (1999) Catabolite regulation of the pta gene as part of carbon flow pathways in Bacillus subtilis. J Bacteriol 181:6889–97
Ratledge C, Holdsworth JE (1985) Properties of a pentulose-5-phosphate phosphoketolase from yeasts grown on xylose. Appl Microbiol Biotechnol 22:217–221
Shin BS, Choi SK, Park SH (1999) Regulation of the Bacillus subtilis phosphotransacetylase gene. J Biochem 126:333–9
Sonderegger M, Schümperli M, Sauer U (2004) Metabolic engineering of a phosphoketolase pathway for pentose catabolism in Saccharomyces cerevisiae. Appl Environ Microbiol 70:2892–7
Spector LB (1980) Acetate kinase: a triple-displacement enzyme. Proc Natl Acad Sci U S A 77:2626–30
Sprague GS Jr (1977) Isolation and characterization of a Saccharomyces cerevisiae mutant deficient in pyruvate kinase activity. J Bacteriol 1:232–41
Thykaer J, Nielsen J (2007) Evidence, through C13-labelling analysis, of phosphoketolase activity in fungi. Process Biochem 42:1050–1055
Toivari MH, Maaheimo H, Penttilä M, Ruohonen L (2010) Enhancing the flux of d-glucose to the pentose phosphate pathway in Saccharomyces cerevisiae for the production of d-ribose and ribitol. Appl Microbiol Biotechnol 85:731–9
Valdés-Hevia MD, de la Guerra R, Gancedo C (1989) Isolation and characterization of the gene encoding phosphoenolpyruvate carboxykinase from Saccharomyces cerevisiae. FEBS Lett 2:313–6
van Winden WA, Wittmann C, Heinzle E, Heijnen JJ (2002) Correcting mass isotopomer distributions for naturally occurring isotopes. Biotechnol Bioeng 80:477–9
Verduyn C, Postma E, Scheffers WA, Van Dijken JP (1992) Effect of benzoic acid on metabolic fluxes in yeasts: a continuous-culture study on the regulation of respiration and alcoholic fermentation. Yeast 8:501–17
Villas-Boas SG, Mas S, Akesson M, Smedsgaard J, Nielsen J (2005) Mass spectrometry in metabolome analysis. Mass Spectrom Rev 24:613–46
Wiechert W (2001) 13C metabolic flux analysis. Metabol Eng 3:195–206
Wittmann C (2007) Fluxome analysis using GC-MS. Microb Cell Fact 6:6
Zamboni N, Fendt SM, Rühl M, Sauer U (2009) (13)C-based metabolic flux analysis. Nat Protoc 4:878–92
Zamboni N, Sauer U (2009) Novel biological insights through metabolomics and 13C-flux analysis. Curr Opin Microbiol 12:553–8
Zelle RM, Trueheart J, Harrison JC, Pronk JT, van Maris AJ (2010) Phosphoenolpyruvate carboxykinase as the sole anaplerotic enzyme in Saccharomyces cerevisiae. Appl Environ Microbiol 16:5383–9
Acknowledgments
The authors acknowledge the Knut and Wallenberg foundation, the Chalmers foundation, and the European Research Council. Saeed Shoaie is acknowledged for the technical support during flux calculations.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary 1
DOCX 13 kb
Rights and permissions
About this article
Cite this article
Papini, M., Nookaew, I., Siewers, V. et al. Physiological characterization of recombinant Saccharomyces cerevisiae expressing the Aspergillus nidulans phosphoketolase pathway: validation of activity through 13C-based metabolic flux analysis. Appl Microbiol Biotechnol 95, 1001–1010 (2012). https://doi.org/10.1007/s00253-012-3936-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-012-3936-0