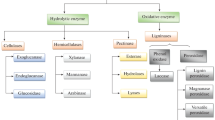
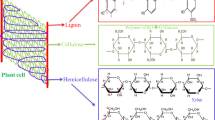
Abstract
The search for petroleum alternatives has motivated intense research into biological breakdown of lignocellulose to produce liquid fuels such as ethanol. Degradation of lignocellulose for biofuel production is a difficult process which is limited by, among other factors, the recalcitrance of lignocellulose and biological toxicity of the products. Consolidated bioprocessing has been suggested as an efficient and economical method of producing low value products from lignocellulose; however, it is not clear whether this would be accomplished more efficiently with a single organism or community of organisms. This review highlights examples of mixtures of microbes in the context of conceptual models for developing symbiotic consortia for biofuel production from lignocellulose. Engineering a symbiosis within consortia is a putative means of improving both process efficiency and stability relative to monoculture. Because microbes often interact and exist attached to surfaces, quorum sensing and biofilm formation are also discussed in terms of consortia development and stability. An engineered, symbiotic culture of multiple organisms may be a means of assembling a novel combination of metabolic capabilities that can efficiently produce biofuel from lignocellulose.




Similar content being viewed by others
References
Agbogbo FK, Coward-Kelly G (2008) Cellulosic ethanol production using the naturally occurring xylose-fermenting yeast, Pichia stipitis. Biotechnol Lett 30:1515–1524. doi:10.1007/s10529-008-9728-z
Alper H, Stephanopoulos G (2009) Engineering for biofuels: exploiting innate microbial capacity or importing biosynthetic potential? Nat Rev Microbiol 7:715–723. doi:10.1038/nrmicro2186
Angenent LT, Wrenn BA (2008) Optimizing mixed-culture bioprocessing to convert wastes into bioenergy. In: Wall JD, Harwood CS, Demain A (eds) bioenergy. ASM, Washington, pp 179–194
Atlas RM (1997) Biodiversity and microbial interactions in the biodegradation of organic compounds. In: Cloete TE, Muyima NYO (eds) Microbial community analysis: the key to the design of biological wastewater treatment systems. IWAQ, London, pp 25–34
Bayer TS, Widmaier DM, Temme K, Ea M, Santi DV, Voigt CA (2009) Synthesis of methyl halides from biomass using engineered microbes. J Am Chem Soc 131:6508–6515. doi:10.1021/ja809461u
Bernstein HC, Paulson SD, Carlson RP (2011) Synthetic Escherichia coli consortia engineered for syntrophy demonstrate enhanced biomass productivity. J Biotechnol. doi:10.1016/j.jbiotec.2011.10.001
Bothast RJ, Saha BC, Flosenzier AV, Ingram LO (1994) Fermentation of L-arabinose, D-xylose and D-glucose by ethanologenic recombinant Klebsiella oxytoca strain P2. Biotechnol Lett 16:401–406
Brenner K, Arnold FH (2011) Self-organization, layered structure, and aggregation enhance persistence of a synthetic biofilm consortium. PLoS One 6:1–7. doi:10.1371/journal.pone.0016791
Brenner K, You L, Arnold FH (2008) Engineering microbial consortia: a new frontier in synthetic biology. Trends Biotechnol 26:483–489. doi:10.1016/j.tibtech.2008.05.004
Breugelmans P, Barken KB, Tolker-Nielsen T, Hofkens J, Dejonghe W, Springael D (2008) Architecture and spatial organization in a triple-species bacterial biofilm synergistically degrading the phenylurea herbicide linuron. FEMS Microbiol Ecol 64:271–282. doi:10.1111/j.1574-6941.2008.00470.x
Brune A (2006) Symbiotic associations between termites and prokaryotes. In: Dworkin M, Falkow S, Rosenberg E, Schleifer K, Stackebrandt E (eds) The prokaryotes. Springer, New York, pp 439–474
Brune A, Ohkuma M (2011) Role of termite gut microbiota in symbiotic digestion. In: Bignell DE (ed) The biology of termites: a modern synthesis. Springer, Dordrecht, pp 439–479
Bugg TD, Ahmad M, Hardiman EM, Singh R (2011) The emerging role for bacteria in lignin degradation and bio-product formation. Curr Opin Biotechnol 22:394–400. doi:10.1016/j.copbio.2010.10.009
Cavedon K, Canale-Parole E (1992) Physiological interactions between a mesophilic cellulolytic Clostridium and a non-cellulolytic bacterium. FEMS Microbiol Lett 86:237–245
Chaffron S, von Mering C (2007) Termites in the woodwork. Genome Biol 8:229–229. doi:10.1186/gb-2007-8-11-229
Choudhary S, Schmidt-Dannert C (2010) Applications of quorum sensing in biotechnology. Appl Microbiol Biotechnol 86:1267–1279. doi:10.1007/s00253-010-2521-7
Costerton JW (1995) Overview of microbial biofilms. J Ind Microbiol 15:137–140
Davey ME, O’toole GA (2000) Microbial biofilms: from ecology to molecular genetics. Microbiol Mol Biol Rev 64:847–867
Dien BS, Nichols NN, O’Bryan PJ, Bothast RJ (2000) Development of new ethanologenic Escherichia coli strains for fermentation of lignocellulosic biomass. Appl Biochem Biotechnol 84–86:181–196
Drysdale GS, Fleet GH (1989) The growth and survival of acetic acid bacteria in wines at different concentrations of oxygen. Am J Enol Vitic 40:99–105
Eiteman MA, Lee SA, Altman E (2008) A co-fermentation strategy to consume sugar mixtures effectively. J Biol Eng 2:3. doi:10.1186/1754-1611-2-3
Feng Y, Yu Y, Wang X, Qu Y, Li D, He W, Kim BH (2011) Degradation of raw corn stover powder (RCSP) by an enriched microbial consortium and its community structure. Bioresour Technol 102:742–747. doi:10.1016/j.biortech.2010.08.074
Fischer CR, Klein-Marcuschamer D, Stephanopoulos G (2008) Selection and optimization of microbial hosts for biofuels production. Metab Eng 10:295–304. doi:10.1016/j.ymben.2008.06.009
Franzén CJ (2003) Metabolic flux analysis of RQ-controlled microaerobic ethanol production by Saccharomyces cerevisiae. Yeast 20:117–132. doi:10.1002/yea.956
Geng A, He Y, Qian C, Yan X, Zhou Z (2010) Effect of key factors on hydrogen production from cellulose in a co-culture of Clostridium thermocellum and Clostridium thermopalmarium. Bioresour Technol 101:4029–4033. doi:10.1016/j.biortech.2010.01.042
Guevara C, Zambrano MM (2006) Sugarcane cellulose utilization by a defined microbial consortium. FEMS Microbiol Lett 255:52–58. doi:10.1111/j.1574-6968.2005.00050.x
Gupta VK, Minocha AK, Jain N (2001) Batch and continuous studies on treatment of pulp mill wastewater by Aeromonas formicans. Chem Technol 552:547–552. doi:10.1002/jctb.417
Haruta S, Cui Z, Huang Z, Li M, Ishii M, Igarashi Y (2002) Construction of a stable microbial community with high cellulose-degradation ability. Appl Microbiol Biotechnol 59:529–534. doi:10.1007/s00253-002-1026-4
Homma H, Shinoyama H, Nobuta Y, Terashima Y, Amachi S, Fujii T (2006) Lignin-degrading activity of edible mushroom Strobilurus ohshimae that forms fruiting bodies on buried sugi (Cryptomeria japonica) twigs. J Wood Sci 53:80–84. doi:10.1007/s10086-006-0810-7
Hoppe GK, Hansford GS (1984) The effect of micro-aerobic conditions on continuous ethanol production by Saccharomyces cerevisiae. Biotechnol Lett 6:681–686
Jayaraman A, Hallock PJ, Carson RM, Lee CC, Mansfeld FB, Wood TK (1999) Inhibiting sulfate-reducing bacteria in biofilms on steel with antimicrobial peptides generated in situ. Appl Microbiol Biotechnol 52:267–275
Jin M, Balan V, Gunawan C, Dale BE (2011) Consolidated bioprocessing (CBP) performance of Clostridium phytofermentans on AFEX-treated corn stover for ethanol production. Biotechnol Bioeng 108:1290–1297. doi:10.1002/bit.23059
Joyeux A, Lafon-Lafourcade S, Ribéreau-Gayon P (1984) Evolution of acetic acid bacteria during fermentation and storage of wine. Appl Environ Microbiol 48:153–156
Kalscheuer R, Stölting T, Steinbüchel A (2006) Microdiesel: Escherichia coli engineered for fuel production. Soc General Microbiol 152:2529–2536. doi:10.1099/mic.0.29028-0
Kato S, Haruta S, Cui ZJ, Ishii M, Igarashi Y (2004) Effective cellulose degradation by a mixed-culture system composed of a cellulolytic Clostridium and aerobic non-cellulolytic bacteria. FEMS Microbiol Ecol 51:133–142. doi:10.1016/j.femsec.2004.07.015
Kato S, Haruta S, Cui ZJ, Ishii M, Igarashi Y (2005) Stable coexistence of five bacterial strains as a cellulose-degrading community. Appl Environ Microbiol 71:7099–7099. doi:10.1128/AEM.71.11.7099
Kim HJ, Boedicker JQ, Choi JW, Ismagilov RF (2008) Defined spatial structure stabilizes a synthetic multispecies bacterial community. PNAS 105:18188–18193. doi:10.1073/pnas.0807935105
Kleerebezem R, van Loosdrecht MCM (2007) Mixed culture biotechnology for bioenergy production. Curr Opin Biotechnol 18:207–212. doi:10.1016/j.copbio.2007.05.001
Klitgord N, Sagré D (2010) Environments that induce synthetic microbial ecosystems. PLoS Comput Biol 6:375–401. doi:10.1371/journal.pcbi.1001002
la Grange DC, den Haan R, van Zyl WH (2010) Engineering cellulolytic ability into bioprocessing organisms. Appl Microbiol Biotechnol 87:1195–1208. doi:10.1007/s00253-010-2660-x
Lawford HG, Rousseau JD, Mohagheghi A, McMillan JD (1999) Fermentation performance characteristics of a prehydrolysate-adapted xylose-fermenting recombinant Zymomonas in batch and continuous fermentations. Appl Biochem Biotechnol 77:191–204
Lee J, Jayaraman A, Wood TK (2007) Indole is an inter-species biofilm signal mediated by SdiA. BMC Microbiol 7:42. doi:10.1186/1471-2180-7-42
Leschine SB (1995) Cellulose degradation in anaerobic environments. Annu Rev Microbiol 49:399–426
Lin Y, Tanaka S (2006) Ethanol fermentation from biomass resources: current state and prospects. Appl Microbiol Biotechnol 69:627–642. doi:10.1007/s00253-005-0229-x
Liu Y, Yu P, Song X, Qu Y (2008) Hydrogen production from cellulose by co-culture of Clostridium thermocellum JN4 and Thermoanaerobacterium thermosaccharolyticum GD17. Int J Hydrogen Energy 33:2927–2933. doi:10.1016/j.ijhydene.2008.04.004
Lu Y, Zhang YP, Lynd LR (2006) Enzyme-microbe synergy during cellulose hydrolysis by Clostridium thermocellum. PNAS 103:16165–16169
Lv Z, Yang J, Wang E, Yuan H (2008) Characterization of extracellular and substrate-bound cellulases from a mesophilic sugarcane bagasse-degrading microbial community. J Gen Appl Microbiol 43:1467–1472. doi:10.1016/j.procbio.2008.08.001
Lynd LR (1996) Overview and evaluation of fuel ethanol from cellulosic biomass: technology, economics, the environment, and policy. Annu Rev Energy Environ 21:403–465. doi:10.1146/annurev.energy.21.1.403
Lynd LR, Weimer PJ, Van Zyl WH, Pretorius IS (2002) Microbial cellulose utilization: fundamentals and biotechnology. Microbiol Molecul Biol Rev 66:506–739. doi:10.1128/MMBR.66.3.506
Maki M, Leung KT, Qin W (2009) The prospects of cellulase-producing bacteria for the bioconversion of lignocellulosic biomass. Intl J Biol Sci 5:500–516
McCarty PL (2007) Thermodynamic electron equivalents model for bacterial yield prediction: modifications and comparative evaluations. Biotechnol Bioeng 97:377–388. doi:10.1002/bit
Miller MB, Bassler BL (2001) Quorum sensing in bacteria. Annu Rev Microbiol 55:165–199
Miyazaki K, Irbis C, Takada J, Matsuura A (2008) An ability of isolated strains to efficiently cooperate in ethanolic fermentation of agricultural plant refuse under initially aerobic thermophilic conditions: oxygen deletion process appended to consolidated bioprocessing (CBP). Bioresour Technol 99:1768–1775. doi:10.1016/j.biortech.2007.03.045
Mohagheghi A, Evans K, Chou Y, Zhang M (2002) Cofermentation of glucose, xylose, and arabinose by genomic DNA-integrated xylose/arabinose fermenting strain of Zymomonas mobilis AX101. Appl Biochem Biotechnol 98–100:885–898
Moons P, Michiels CW, Aertsen A (2009) Bacterial interactions in biofilms. Crit Rev Microbiol 35:157–168. doi:10.1080/10408410902809431
Nakashimada Y, Srinivasan K, Murakami M, Nishio N (2000) Direct conversion of cellulose to methane by anaerobic fungus Neocallimastix frontalis and defined methanogens. Biotechnol Lett 22:223–227
Ohta K, Beall DS, Mejia JP, Shanmugam KT, Ingram LO (1991) Metabolic engineering of Klebsiella oxytoca M5A1 for ethanol production from xylose and glucose. Appl Environ Microbiol 57:2810–2815
Okeke BC, Lu J (2011) Characterization of a defined cellulolytic and xylanolytic bacterial consortium for bioprocessing of cellulose and hemicelluloses. Appl Biochem Biotechnol 163:869–881. doi:10.1007/s12010-010-9091-0
Olsson L, Hahn-Hagerdal B (1996) Fermentation of lignocellulosic hydrolysates for ethanol production. Enzyme Microb Technol 18:312–331. doi:10.1016/0141-0229(95)00157-3
Paerl HW, Pinckney JL (1996) A mini-review of microbial consortia: their roles in aquatic production and biogeochemical cycling. Microb Ecol 31:225–247
Pavlostathis SG, Miller TL, Wolin MJ (1990) Cellulose fermentation by continuous cultures of Ruminococcus albus and Methanobrevibacter smithii. Appl Microbiol Biotechnol 33:109–116
Roeder J, Schink B (2009) Syntrophic degradation of cadaverine by a defined methanogenic co-culture. Appl Environ Microbiol 75:4821–4828. doi:10.1128/AEM.00342-09
Roos W, Luckner M (1984) Relationships between proton extrusion and fluxes of ammonium ions and organic acids in Penicillium cyclopium. J Gen Microbiol 130:1007–1014. doi:10.1099/00221287-130-4-1007
Rosche B, Li XZ, Hauer B, Schmid A, Buehler K (2009) Microbial biofilms: a concept for industrial catalysis? Trends Biotechnol 27:636–643. doi:10.1016/j.tibtech.2009.08.001
Rouland-Lefévre C, Bignell D (2004) Cultivation of symbiotic fungi by termites of the subfamily Macrotermitinae. Symbiosis 4:731–756. doi:10.1007/0-306-48173-1_46
Sánchez C (2009) Lignocellulosic residues: biodegradation and bioconversion by fungi. Biotechnol Adv 27:185–194. doi:10.1016/j.biotechadv.2008.11.001
Scharf ME, Tartar A (2008) Termite digestomes as sources for novel lignocellulases. Biofuels, Bioprod Biorefining 2:540–552. doi:10.1002/bbb
Schink B (1997) Energetics of syntrophic cooperation in methanogenic degradation. Microbiol Molecul Biol Rev 61:262–262
Shaw JA, Podkaminer KK, Desai SG, Bardsley JS, Rogers SR, Thorne PG, Hogsett DA, Lynd LR (2008) Metabolic engineering of a thermophilic bacterium to produce ethanol at high yield. PNAS 105:13769–13774. doi:10.1073/pnas.0801266105
Shin H, McClendon S, Vo T, Chen RR (2010) Escherichia coli binary culture engineered for direct fermentation of hemicellulose to a biofuel. Appl Environ Microbiol 76:8150–8159. doi:10.1128/AEM.00908-10
Shou W, Ram S, Vilar JMG (2007) Synthetic cooperation in engineered yeast populations. PNAS 104:1877–1882. doi:10.1073/pnas.0610575104
Stewart PS, Franklin MJ (2008) Physiological heterogeneity in biofilms. Nat Rev Microbiol 6:199–210. doi:10.1038/nrmicro1838
Szambelan K, Nowak J, Czarnecki Z (2004) Use of Zymomonas mobilis and Saccharomyces cerevisiae mixed with Kluyveromyces fragilis for improved ethanol production from Jerusalem artichoke tubers. Biotechnol Lett 26:845–848
Tolker-Nielsen T, Molin S (2000) Spatial organization of microbial biofilm communities. Microb Ecol 40:75–84. doi:10.1007/s002480000057
Veal DA, Lynch JM (1984) Associative cellulolysis and dinitrogen fixation by co-cultures of Trichoderma harzianum and Clostridium butyricum. Nature 310:695–696
Vega J, Clausen E, Gaddy J (1988) Biofilm reactors for ethanol production. Enzyme Microb Technol 10:390–402. doi:10.1016/0141-0229(88)90033-6
Wang Z, Chen S (2009) Potential of biofilm-based biofuel production. Appl Microbiol Biotechnol 83:1–18. doi:10.1007/s00253-009-1940-9
Wang A, Ren N, Shi Y, Lee D (2008) Bioaugmented hydrogen production from microcrystalline cellulose using co-culture of Clostridium acetobutylicum X9 and Ethanoigenens harbinense B49. Int J Hydrog Energy 33:912–917. doi:10.1016/j.ijhydene.2007.10.017
Wang W, Yan L, Cui Z, Gao Y, Wang Y, Jing R (2011) Characterization of a microbial consortium capable of degrading lignocellulose. Bioresour Technol 102(19):9321–9324. doi:10.1016/j.biortech.2011.07.065
Warikoo V, McInerney MJ, Robinson JA, Suflita JM (1996) Interspecies acetate transfer influences the extent of anaerobic benzoate degradation by syntrophic consortia. Appl Environ Microbiol 62:26–32
Warnick TA, Methé BA and Leschine SB (2002) Clostridium phytofermentans sp. nov., a cellulolytic mesophile from forest soil. Int J Syst Evol Microbiol 52:1155–1160
Warnecke F, Luginühl P, Ivanova N, Ghassemian M, Richardson TH, Stege JT, Cayouette M, McHardy AC, Djordjevic G, Aboushadi N, Sorek R, Tringe SG, Podar M, Martin HG, Kunin V, Dalevi D, Madejska J, Kirton E, Platt D, Szeto E, Salamov A, Barry K, Mikhailova N, Kyrpides NC, Matson EG, Ottesen EA, Zhang X, Hernández M, Murillo C, Acosta LG, Rigoutsos I, Tamayo G, Green BD, Chang C, Rubin EM, Mathur EJ, Robertson DE, Hugenholtz P, Leadbetter JR (2007) Metagenomic and functional analysis of hindgut microbiota of a wood-feeding higher termite. Nature 450:560–565. doi:10.1038/nature06269
Wongwilaiwalin S, Rattanachomsri U, Laothanachareon T, Eurwilaichitr L, Igarashi Y, Champreda V (2010) Analysis of a thermophilic lignocellulose degrading microbial consortium and multi-species lignocellulolytic enzyme system. Enzyme Microb Technol 47:283–290. doi:10.1016/j.enzmictec.2010.07.013
Wood TK, Hong SH, Ma Q (2010) Engineering biofilm formation and dispersal. Trends Biotechnol 29:87–94. doi:10.1016/j.tibtech.2010.11.001
Wyman CE (1996) Ethanol production from lignocellulosic biomass: overview. In: Wyman CE (ed) Handbook on bioethanol: production and utilization. Taylor and Francis, Washington, pp 1–16
Xavier JB, Picioreanu C, van Loosdrecht MCM (2005) A framework for multidimensional modelling of activity and structure of multispecies biofilms. Environ Microbiol 7:1085–1103. doi:10.1111/j.1462-2920.2005.00787.x
Xu L, Tschirner U (2011) Improved ethanol production from various carbohydrates through anaerobic thermophilic co-culture. Bioresour Technol 102:10065–10071. doi:10.1016/j.biortech.2011.08.067
Yan J, Op den Camp HJM, Jetten MSM, Hu YY, Haaijer SCM (2010) Induced cooperation between marine nitrifiers and anaerobic ammonium-oxidizing bacteria by incremental exposure to oxygen. Syst Appl Microbiol 33:407–415. doi:10.1016/j.syapm.2010.08.003
You L, Cox RS, Weiss R, Arnold FH (2004) Programmed population control by cell-cell communication and regulated killing. Nature 428:868–871. doi:10.1038/nature02468.1
Zientz E, Dandekar T, Gross R (2004) Metabolic interdependence of obligate intracellular bacteria and their insect hosts. Microbiol Molecul Biol Rev 68:745–770. doi:10.1128/MMBR.68.4.745
Acknowledgments
We would like to thank T.K. Wood at The Pennsylvania State University for his assistance in initial drafts of this work. This work was supported by the National Science Foundation Graduate Research Fellowship under Grant no. DGE-0750756. The authors also acknowledge the financial support to T.R. Zuroff from the John D. and Jeanette McWhirter Fellowship from the Pennsylvania State University Department of Chemical Engineering.
Conflicts of interest
The authors declare they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zuroff, T.R., Curtis, W.R. Developing symbiotic consortia for lignocellulosic biofuel production. Appl Microbiol Biotechnol 93, 1423–1435 (2012). https://doi.org/10.1007/s00253-011-3762-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-011-3762-9