Abstract
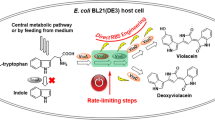
Violacein is a bacteria-originated indolocarbazole pigment with potential applications due to its various bioactivities such as anti-tumor, antiviral, and antifungal activities. However, stable mass production of this pigment is difficult due to its low productivities and the instability of wild-type violacein-producing strains. In order to establish a stable and efficient production system for violacein, the violacein synthesis pathway from a new species of Duganella sp. B2 was reconstructed in different bacterial strains including Escherichia coli, Citrobacter freundii, and Enterobacter aerogenes by using different vectors. The gene cluster that encodes five enzymes involved in the violacein biosynthetic pathway was first isolated from Duganella sp. B2, and three recombinant expression vectors were constructed using the T7 promoter or the alkane-responsive promoter PalkB. Our results showed that violacein could be stably synthesized in E. coli, C. freundii, and E. aerogenes. Interestingly, we found that there were great differences between the different recombinant strains, not only in the protein expression profiles pertaining to violacein biosynthesis but also in the productivity and composition of crude violacein. Among the host strains tested, the crude violacein production by the recombinant C. freundii strain reached 1.68 g L−1 in shake flask cultures, which was 4-fold higher than the highest production previously reported in flask culture by other groups. To the best of our knowledge, this is the first report on the efficient production of violacein by genetically engineered strains.





Similar content being viewed by others

References
Ahmed AM, Shimamoto T (2008) Emergence of a cefepime- and cefpirome-resistant Citrobacter freundii clinical isolate harbouring a novel chromosomally encoded AmpC beta-lactamase, CMY-37. Int J Antimicrob Agents 32(3):256–261
Antonio RV, Creczynski-Pasa TB (2004) Genetic analysis of violacein biosynthesis by Chromobacterium violaceum. Genet Mol Res 3(1):85–91
August PR, Grossman TH, Minor C, Draper MP, MacNeil IA, Pemberton JM, Call KM, Holt D, Osburne MS (2000) Sequence analysis and functional characterization of the violacein biosynthetic pathway from Chromobacterium violaceum. J Mol Microbiol Biotechnol 2(4):513–519
Brady SF, Chao CJ, Handelsman J, Clardy J (2001) Cloning and heterologous expression of a natural product biosynthetic gene cluster from eDNA. Org Lett 3(13):1981–1984
Bromberg Natalia DN (2001) Violacein transformation by peroxidases and oxidases: implications on its biological properties. J Mol Catal B Enzym 11:463–467
Conrado RJ, Varner JD, DeLisa MP (2008) Engineering the spatial organization of metabolic enzymes: mimicking nature’s synergy. Curr Opin Biotechnol 19(5):492–499
Creczynski-Pasa TB, Antonio RV (2004) Energetic metabolism of Chromobacterium violaceum. Genet Mol Res 3(1):162–166
Daniel R, Stuertz K, Gottschalk G (1995) Biochemical and molecular characterization of the oxidative branch of glycerol utilization by Citrobacter freundii. J Bacteriol 177(15):4392–4401
De Almeida DF, Hungria M, Guimaraes CT, Antonio RV, Almeida FC, de Almeida LGP, de Almeida R, Alves-Gomes JA, Andrade EM, Araripe J (2003) The complete genome sequence of Chromobacterium violaceum reveals remarkable and exploitable bacterial adaptability. Proc Natl Acad Sci USA 100(20):11660–11665
Demoss RD, Evans NR (1960) Incorporation of C14-labeled substrates into violacein. J Bacteriol 79:729–733
Durán N, Antonio RV, Haun M, Pilli RA (1994) Biosynthesis of a trypanocide by Chromobacterium violaceum. World J Microbiol Biotechnol V10(6):686–690
Durán N, Erazo S, Campos V (1983) Bacterial Chemistry-II: antimicrobial photoproduct from pigment of Chromobacterium violaceum. An Acad Bras Ciênc 55:231–234
Duran N, Justo GZ, Ferreira CV, Melo PS, Cordi L, Martins D (2007) Violacein: properties and biological activities. Biotechnol Appl Biochem 48:127–133
Duran N, Menck CF (2001) Chromobacterium violaceum: a review of pharmacological and industiral perspectives. Crit Rev Microbiol 27(3):201–222
Gillis M, Ley J (2006) The Genera Chromobacterium and Janthinobacterium. The Prokaryotes 5:737–746
Hirano S, Asamizu S, Onaka H, Shiro Y, Nagano S (2008) Crystal structure of VioE, a key player in the construction of the molecular skeleton of violacein. J Biol Chem 283(10):6459–6466
John M, Pemberton KMV, Penfold RJ (1991) Cloning and heterologous expression of the violacein biosynthesis gene cluster from Chromobacterium violaceum. Curr Microbiol 22(6):355–358
Leon LL, Miranda CC, De Souza AO, Duran N (2001) Antileishmanial activity of the violacein extracted from Chromobacterium violaceum. J Antimicrob Chemother 48(3):449–450
Lu Y, Wang L, Xue Y, Zhang C, Xing X-H, Lou K, Zhang Z, Li Y, Zhang G, Bi J (2009) Production of violet pigment by a newly isolated psychrotrophic bacterium from a glacier in Xinjiang, China. Biochem Eng J 43(2):135–141
Manukhov IV, Mamaeva DV, Morozova EA, Rastorguev SM, Faleev NG, Demidkina TV, Zavilgelsky GB (2006) l-methionine gamma-lyase from Citrobacter freundii: cloning of the gene and kinetic parameters of the enzyme. Biochemistry (Moscow) 71(4):361–369
Matz C, Deines P, Boenigk J, Arndt H, Eberl L, Kjelleberg S, Jurgens K (2004) Impact of violacein-producing bacteria on survival and feeding of bacterivorous nanoflagellates. Appl Environ Microbiol 70(3):1593–1599
Matz C, Webb JS, Schupp PJ, Phang SY, Penesyan A, Egan S, Steinberg P, Kjelleberg S (2008) Marine biofilm bacteria evade eukaryotic predation by targeted chemical defense. PLoS ONE 3(7):e2744
Mendes AS, de Carvalho JE, Duarte MCT, Duran N, Bruns RE (2001) Factorial design and response surface optimization of crude violacein for Chromobacterium violaceum production. Biotechnol Lett 23:1963–1969
Moss MO, Ryall C, Logan NA (1978) The classification and characterization of chromobacteria from a lowland river. J Gen Microbiol 105:11–21
Nakamura ST, Morita Y, Tamiya E (2002) Isolation of a psychrotrophic bacterium from the organic residue of a water tank keeping rainbow trout and antibacterial effect of violet pigment produced from the strain. Biochem Eng J 12(1):79–86
Pantanella F, Berlutti F, Passariello C, Sarli S, Morea C, Schippa S (2007) Violacein and biofilm production in Janthinobacterium lividum. J Appl Microbiol 102(4):992–999
Rettori D, Durán N (1998) Production, extraction and purificationof violacein: an antibiotic pigment producedby Chromobacterium violaceum. World J Microbiol Biotechnol V14(5):685–688
Sanchez C, Brana AF, Mendez C, Salas JA (2006) Reevaluation of the violacein biosynthetic pathway and its relationship to indolocarbazole biosynthesis. ChemBioChem 7(8):1231–1240
Shinoda K, Hasegawa T, Sato H, Shinozaki M, Kuramoto H, Takamiya Y, Sato T, Nikaidou N, Watanabe T, Hoshino T (2007) Biosynthesis of violacein: a genuine intermediate, protoviolaceinic acid, produced by VioABDE, and insight into VioC function. Chem Commun (Camb) (40):4140–4142
Shirata ATT, Yasui H, Hata T, Hayasaka S, Kojima A, Kato H (2000) Isolation of bacteria producing bluish-purple pigment and use for dyeing. JARQ. Jpn Agric Res Q 34(2):131–140
Smits TH, Seeger MA, Witholt B, van Beilen JB (2001) New alkane-responsive expression vectors for Escherichia coli and pseudomonas. Plasmid 46(1):16–24
Srinivasan V, Nam HM, Sawant AA, Headrick SI, Nguyen LT, Oliver SP (2008) Distribution of tetracycline and streptomycin resistance genes and class 1 integrons in Enterobacteriaceae isolated from dairy and nondairy farm soils. Microb Ecol 55(2):184–193
Wang HS, Jiang PX, Lu Y, Ruan ZY, Jiang RB, Xing XH, Lou K, Wei D (2009) Optimization of culture conditions for violacein production by a new strain of Duganella sp. B2. Biochem Eng J 44(2-3):119–124
Wang HS, Lu Y, Xue Y, Ruan ZY, Jiang RB, Xing XH, Lou K, Wei D (2008) Separation, purification and structure identification of purple pigments from Duganella sp. B2. J Chem Ind Eng 59:630–635
Wilkinson B, Micklefield J (2007) Mining and engineering natural-product biosynthetic pathways. Nat Chem Biol 3(7):379–386
Yada S, Wang Y, Zou Y, Nagasaki K, Hosokawa K, Osaka I, Arakawa R, Enomoto K (2008) Isolation and characterization of two groups of novel marine bacteria producing violacein. Mar Biotechnol (NY) 10(2):128–132
Yang LH, Xiong H, Lee OO, Qi SH, Qian PY (2007) Effect of agitation on violacein production in Pseudoalteromonas luteoviolacea isolated from a marine sponge. Lett Appl Microbiol 44(6):625–630
Yoshitoshi Nakamura CA, Tatsuro S (2003) Production of antibacterial violet pigment by psychrotropic bacterium RT102 strain. Biotechnol Bioprocess Eng 8:37–40
Acknowledgements
The authors thank Prof. B. Witholt of ETH, Swiss for kindly donating the plasmid pCom10. This work was supported in part by the National Science Fund of China (Grant No. 20676071 and 20836004), China Postdoctoral Science Foundation funded project (Grant No. 023206061) and Xinjiang-supporting project by Science and Technology (Grant No. 200991132).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jiang, Px., Wang, Hs., Zhang, C. et al. Reconstruction of the violacein biosynthetic pathway from Duganella sp. B2 in different heterologous hosts. Appl Microbiol Biotechnol 86, 1077–1088 (2010). https://doi.org/10.1007/s00253-009-2375-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-009-2375-z