Abstract
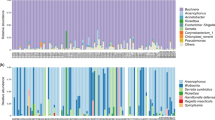
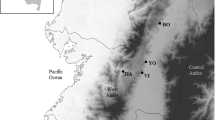
Aphids are well known for their association with endosymbiont bacteria. Almost all aphids harbor Buchnera aphidicola as an obligate symbiont and several other bacteria as facultative symbionts. Associations of facultative symbionts and aphids are quite variable in terms of diversity and prevalence across aphid species. Facultative symbionts can have a major impact on aphid bioecological traits. A number of factors shape the outcome of the facultative symbiont-aphid association, including aphid clone, bacterial genotype, geography, and host plant association. The effects of host plant on aphid-facultative symbiont associations are the least understood. We performed deep sequencing of the bacterial community associated with field populations of the oligophagous aphid Aphis (Toxoptera) citricidus collected from different host plants. We demonstrate that (i) A. citricidus has low symbiont diversity, (ii) symbiont diversity is affected by host plant, and (iii) host plants affect the relative abundance of the obligate symbiont Buchnera and an unknown genus of Enterobacteriaceae.



Similar content being viewed by others
References
Moran NA, McCutcheon JP, Nakabachi A (2008) Genomics and evolution of heritable bacterial symbionts. Annu Rev Genet 42(1):165–190
Douglas AE (1998) Nutritional interactions in insect-microbial symbioses: aphids and their symbiotic bacteria Buchnera. Annu Rev Entomol 43:17–37
Douglas AE (2009) The microbial dimension in insect nutritional ecology. Funct Ecol 23(1):38–47
Hansen AK, Moran NA (2014) The impact of microbial symbionts on host plant utilization by herbivorous insects. Mol Ecol 23(6):1473–1496
Oliver KM, Moran NA, Hunter MS (2005) Variation in resistance to parasitism in aphids is due to symbionts not host genotype. Proc Natl Acad Sci U S A 102(36):12795–12800
Oliver KM, Noge K, Huang EM, Campos JM, Becerra JX, Hunter MS (2012) Parasitic wasp responses to symbiont-based defense in aphids. BMC Biol 10:11
Oliver KM, Russell JA, Moran NA, Hunter MS (2003) Facultative bacterial symbionts in aphids confer resistance to parasitic wasps. Proc Natl Acad Sci U S A 100(4):1803–1807
Oliver KM, Campos J, Moran NA, Hunter MS (2008) Population dynamics of defensive symbionts in aphids. Proc Biol Sci R Soc 275(1632):293–299
Montllor CB, Maxmen A, Purcell AH (2002) Facultative bacterial endosymbionts benefit pea aphids Acyrthosiphon pisum under heat stress. Ecol Entomol 27(2):189–195
Scarborough CL, Ferrari J, Godfray HC (2005) Aphid protected from pathogen by endosymbiont. Science 310(5755):1781
Łukasik P, van Asch M, Guo H, Ferrari J, Charles J, Godfray H (2013) Unrelated facultative endosymbionts protect aphids against a fungal pathogen. Ecol Lett 16(2):214–218
Su Q, Zhou X, Zhang Y (2013) Symbiont-mediated functions in insect hosts. Commun Integr Biol 6(3):e23804
Tsuchida T, Koga R, Fujiwara A, Fukatsu T (2014) Phenotypic effect of “Candidatus Rickettsiella viridis,” a facultative symbiont of the pea aphid (Acyrthosiphon pisum), and its interaction with a coexisting symbiont. Appl Environ Microbiol 80(2):525–533
Tsuchida T, Koga R, Horikawa M, Tsunoda T, Maoka T, Matsumoto S, Simon JC, Fukatsu T (2010) Symbiotic bacterium modifies aphid body color. Science 330:1102–1104
Fukatsu T, Tsuchida T, Nikoh N, Koga R (2001) Spiroplasma symbiont of the pea aphid, Acyrthosiphon pisum (Insecta: Homoptera). Appl Environ Microbiol 67(3):1284–1291
Tsuchida T, Koga R, Shibao H, Matsumoto T, Fukatsu T (2002) Diversity and geographic distribution of secondary endosymbiotic bacteria in natural populations of the pea aphid, Acyrthosiphon pisum. Mol Ecol 11(10):2123–2135
Haynes S, Darby AC, Daniell TJ, Webster G, Van Veen FJ, Godfray HC, Prosser JI, Douglas AE (2003) Diversity of bacteria associated with natural aphid populations. Appl Environ Microbiol 69(12):7216–7223
Burke GR, Normark BB, Favret C, Moran NA (2009) Evolution and diversity of facultative symbionts from the aphid subfamily Lachninae. Appl Environ Microbiol 75(16):5328–5335
Clark EL, Daniell TJ, Wishart J, Hubbard SF, Karley AJ (2012) How conserved are the bacterial communities associated with aphids? A detailed assessment of the Brevicoryne brassicae (Hemiptera: Aphididae) using 16S rDNA. Environ Entomol 41(6):1386–1397
Sevim E, Çelebi Ö, Sevim A (2012) Determination of the bacterial flora as a microbial control agent of Toxoptera aurantii (Homoptera: Aphididae). Biologia 67(2):397–404
Brady CM, Asplen MK, Desneux N, Heimpel GE, Hopper KR, Linnen CR, Oliver KM, Wulff JA, White JA (2014) Worldwide populations of the aphid Aphis craccivora are infected with diverse facultative bacterial symbionts. Microb Ecol 67(1):195–204
Ferrari J, West JA, Via S, Godfray HC (2012) Population genetic structure and secondary symbionts in host-associated populations of the pea aphid complex. Evol Int J Org Evol 66(2):375–390
Russell JA, Weldon S, Smith AH, Kim KL, Hu Y, Lukasik P, Doll S, Anastopoulos I, Novin M, Oliver KM (2013) Uncovering symbiont-driven genetic diversity across North American pea aphids. Mol Ecol 22(7):2045–2059
Henry LM, Peccoud J, Simon JC, Hadfield JD, Maiden MJ, Ferrari J, Godfray HC (2013) Horizontally transmitted symbionts and host colonization of ecological niches. Curr Biol 23(17):1713–1717
McLean AHC, van Asch M, Ferrari J, Godfray HCJ (2011) Effects of bacterial secondary symbionts on host plant use in pea aphids. Proc R Soc B Biol Sci 278(1706):760–766
Via S (1991) Specialized host plant performance of pea aphid clones is not altered by experience. Ecology 72(4):1420–1427
Leonardo TE, Muiru GT (2003) Facultative symbionts are associated with host plant specialization in pea aphid populations. Proc R Soc B Biol Sci 270:209–212
Tsuchida T, Koga R, Fukatsu T (2004) Host plant specialization governed by facultative symbiont. Science 303(5666):1989
Leonardo TE (2004) Removal of a specialization-associated symbiont does not affect aphid fitness. Ecol Lett 7(6):461–468
Brady CM, White JA (2013) Cowpea aphid (Aphis craccivora) associated with different host plants has different facultative endosymbionts. Ecol Entomol 38(4):433–437
Michaud JP (1998) A review of the literature on Toxoptera citricida (Kirkaldy) (Homoptera : Aphididae). Fla Entomol 81(1):37–61
Halbert SE, Brown LG (1996) Toxoptera citricida (Kirkaldy), brown citrus aphid—identification, biology and management strategies. Fla Dep Agric Consum Serv Div Plant Ind Entomol Circ 374:6
Sunnucks P, De Barro PJ, Lushai G, Maclean N, Hales D (1997) Genetic structure of an aphid studied using microsatellites: cyclic parthenogenesis, differentiated lineages and host specialization. Mol Ecol 6(11):1059–1073
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI et al (2010) QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7(5):335–336
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30(12):2725–2729
Russell JA, Latorre A, Sabater-Munoz B, Moya A, Moran NA (2003) Side-stepping secondary symbionts: widespread horizontal transfer across and beyond the Aphidoidea. Mol Ecol 12(4):1061–1075
Ferrari J, Darby AC, Daniell TJ, Godfray HCJ, Douglas AE (2004) Linking the bacterial community in pea aphids with host-plant use and natural enemy resistance. Ecol Entomol 29(1):60–65
Jones RT, Bressan A, Greenwell AM, Fierer N (2011) Bacterial communities of two parthenogenetic aphid species cocolonizing two host plants across the Hawaiian Islands. Appl Environ Microbiol 77(23):8345–8349
Russell JA, Moran NA (2006) Costs and benefits of symbiont infection in aphids: variation among symbionts and across temperatures. Proc Biol Sci R Soc 273(1586):603–610
Guay JF, Boudreault S, Michaud D, Cloutier C (2009) Impact of environmental stress on aphid clonal resistance to parasitoids: role of Hamiltonella defensa bacterial symbiosis in association with a new facultative symbiont of the pea aphid. J Insect Physiol 55(10):919–926
Nyabuga FN, Outreman Y, Simon J-C, Heckel DG, Weisser WW (2010) Effects of pea aphid secondary endosymbionts on aphid resistance and development of the aphid parasitoid Aphidius ervi: a correlative study. Entomol Exp Appl 136(3):243–253
Leonardo TE, Mondor EB (2006) Symbiont modifies host life-history traits that affect gene flow. Proc Biol Sci R Soc 273(1590):1079–1084
Werren JH, Baldo L, Clark M (2008) Wolbachia: master manipulators of invertebrate biology. Nat Rev Microbiol 6(10):741–751
Jeyaprakash A, Hoy MA (2000) Long PCR improves Wolbachia DNA amplification: wsp sequences found in 76% of sixty-three arthropod species. Insect Mol Biol 9(4):393–405
Gomez-Valero L, Soriano-Navarro M, Perez-Brocal V, Heddi A, Moya A, Garcia-Verdugo JM, Latorre A (2004) Coexistence of Wolbachia with Buchnera aphidicola and a secondary symbiont in the aphid Cinara cedri. J Bacteriol 186(19):6626–6633
Wang Z, Shen Z-R, Song Y, Liu H-Y, Li Z-X (2009) Distribution and diversity of Wolbachia in different populations of the wheat aphid Sitobion miscanthi (Hemiptera: Aphididae) in China. Eur J Entomol 106(1):49–55
Augustinos AA, Santos-Garcia D, Dionyssopoulou E, Moreira M, Papapanagiotou A, Scarvelakis M, Doudoumis V, Ramos S, Aguiar AF, Borges PAV et al (2011) Detection and characterization of Wolbachia infections in natural populations of aphids: is the hidden diversity fully unraveled? PLoS One 6(12):e28695
Wagner SM, Martinez AJ, Ruan Y-M, Kim KL, Lenhart PA, Dehnel AC, Oliver KM, White JA (2015) Facultative endosymbionts mediate dietary breadth in a polyphagous herbivore. Funct Ecol 29(11):1402–1410
Gauthier J-P, Outreman Y, Mieuzet L, Simon J-C (2015) Bacterial communities associated with host-adapted populations of pea aphids revealed by deep sequencing of 16S ribosomal DNA. PLoS One 10(3):e0120664
Najar-Rodriguez AL, McGraw EA, Mensah RK, Pittman GW, Walter GH (2009) The microbial flora of Aphis gossypii: patterns across host plants and geographical space. J Invertebr Pathol 100(2):123–126
Toju H, Fukatsu T (2011) Diversity and infection prevalence of endosymbionts in natural populations of the chestnut weevil: relevance of local climate and host plants. Mol Ecol 20(4):853–868
Zytynska SE, Weisser WW (2016) The natural occurrence of secondary bacterial symbionts in aphids. Ecol Entomol 41(1):13–26
Tsai JH (1998) Development, survivorship, and reproduction of Toxoptera citricida (Kirkaldy) (Homoptera : Aphididae) on eight host plants. Environ Entomol 27(5):1190–1195
Michaud JP (2004) Assessment of cotton as an alternative host plant for the brown citrus aphid, Toxoptera citricida (Homoptera : Aphididae). Fla Entomol 87(2):105–111
Federici CT, Fang DQ, Scora RW, Roose ML (1998) Phylogenetic relationships within the genus Citrus (Rutaceae) and related genera as revealed by RFLP and RAPD analysis. Theor Appl Genet 96(6–7):812–822
Nicolosi E, Deng ZN, Gentile A, La Malfa S, Continella G, Tribulato E (2000) Citrus phylogeny and genetic origin of important species as investigated by molecular markers. Theor Appl Genet 100(8):1155–1166
Barkley NA, Roose ML, Krueger RR, Federici CT (2006) Assessing genetic diversity and population structure in a citrus germplasm collection utilizing simple sequence repeat markers (SSRs). Theor Appl Genet 112(8):1519–1531
Pang X-M, Hu C-G, Deng X-X (2007) Phylogenetic relationships within Citrus and its related genera as inferred from AFLP markers. Genet Resour Crop Evol 54(2):429–436
Carbonell-Caballero J, Alonso R, Ibanez V, Terol J, Talon M, Dopazo J (2015) A phylogenetic analysis of 34 chloroplast genomes elucidates the relationships between wild and domestic species within the genus citrus. Mol Biol Evol 32(8):2015–2035
Froelicher Y, Mouhaya W, Bassene J-B, Costantino G, Kamiri M, Luro F, Morillon R, Ollitrault P (2011) New universal mitochondrial PCR markers reveal new information on maternal citrus phylogeny. Tree Genet Genomes 7(1):49–61
Federici CT, Roose ML, Scora RW (2000) RFLP analysis of the origin of Citrus bergamia, Citrus jambhiri, and Citrus limonia. Proc First Int Symp Citrus Biotechnol 535:55–62
Gosalbes MJ, Latorre A, Lamelas A, Moya A (2010) Genomics of intracellular symbionts in insects. Int J Med Microbiol 300(5):271–278
Koga R, Tsuchida T, Fukatsu T (2003) Changing partners in an obligate symbiosis: a facultative endosymbiont can compensate for loss of the essential endosymbiont Buchnera in an aphid. Proc Biol Sci R Soc 270(1533):2543–2550
Sakurai M, Koga R, Tsuchida T, Meng XY, Fukatsu T (2005) Rickettsia symbiont in the pea aphid Acyrthosiphon pisum: novel cellular tropism, effect on host fitness, and interaction with the essential symbiont Buchnera. Appl Environ Microbiol 71(7):4069–4075
Myers SP (2004) The causes of intestinal dysbiosis: a review. Altern Med Rev 9(2):180–197
Hamdi C, Balloi A, Essanaa J, Crotti E, Gonella E, Raddadi N, Ricci I, Boudabous A, Borin S, Manino A et al (2011) Gut microbiome dysbiosis and honeybee health. J Appl Entomol 135(7):524–533
Minard G, Mavingui P, Moro CV (2013) Diversity and function of bacterial microbiota in the mosquito holobiont. Parasit Vectors 6(146):1–12
Ma D, Storelli G, Mitchell M, Leulier F (2015) Studying host-microbiota mutualism in Drosophila: harnessing the power of gnotobiotic flies. Biomed J 38(4):285–293
Cassone BJ, Redinbaugh MG, Dorrance AE, Michel AP (2015) Shifts in Buchnera aphidicola density in soybean aphids (Aphis glycines) feeding on virus-infected soybean. Insect Mol Biol 24(4):422–431
Acknowledgments
Authors thank FAPESP by providing a PhD fellowship to ASG (Grant # 2012/04287-0, São Paulo Research Foundation). Authors also thank CNPq (Grant # 490474/2011-0) and FAPESP (Grant # 2011/50877-0, São Paulo Research Foundation) for providing research funds to the senior author FLC. Authors are also thankful to Prof. Celso Omoto for allowing the use of a Workstation and MSc. Antonio Rogerio B do Nascimento for providing assistance with software implementation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guidolin, A.S., Cônsoli, F.L. Symbiont Diversity of Aphis (Toxoptera) citricidus (Hemiptera: Aphididae) as Influenced by Host Plants. Microb Ecol 73, 201–210 (2017). https://doi.org/10.1007/s00248-016-0892-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-016-0892-8