Abstract
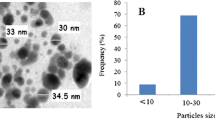
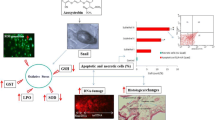
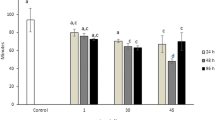
The apoptotic and genotoxic potential of titanium dioxide nanoparticles (TiO2NPs) were evaluated in hemocyte cells of freshwater snail Lymnea luteola L. Before evaluation of the toxic potential, mean size of the TiO2NPs was determined using a transmission electron microscopy and dynamic light scattering. In this study, L. luteola were exposed to different concentrations of TiO2NPs (28, 56, and 84 μg/ml) over 96 h. Induction of oxidative stress in hemolymph was observed by a decrease in reduced glutathione and glutathione-S-transferase levels at different concentration of TiO2NPs and, in contrast, an increase in malondialdehyde and reactive oxygen species levels. Catalase activity was decreased at lower concentrations but increased at greater concentration of TiO2NPs. The extent of DNA fragmentation occurring in L. luteola due to ecotoxic impact TiO2NPs was further substantiated by alkaline single-cell gel electrophoresis assay and expressed in terms of % tail DNA and olive tail moment. The alkaline single-cell gel electrophoresis assay for L. luteola clearly shown relatively greater DNA damage at the highest concentration of TiO2NPs.The results indicate that the interaction of TiO2NPs with snail influences toxicity, which is mediated by oxidative stress according dose and in a time-dependent manner. The results of this study showed the importance of a multibiomarker approach for assessing the injurious effects of TiO2NPs to freshwater snail L. luteola, which may be vulnerable due to the continuous discharge of TiO2NPs into the aquatic ecosystems. The measurement of DNA integrity in L. luteola thus provides an early warning signal of contamination of the aquatic ecosystem by TiO2NPs.






Similar content being viewed by others
References
Ali D, Nagpure NS, Kumar S, Kumar R, Kushwaha B (2008) Genotoxicity assessment of acute exposure of chlorpyrifos to freshwater fish Channa punctatus (Bloch) using micronucleus assay and alkaline single-cell gel electrophoresis. Chemosphere 71:1823–1831
Ali D, Alarifi S, Kumar S, Ahamed M, Siddiqui MA (2012) Oxidative stress and genotoxic effect of zinc oxide nanoparticles in freshwater snail Lymnaea luteola L. Aquat Toxicol 124–125:83–90
Ali D, Yadav PG, Kumar S, Ali H, Alarifi S, Harrath AH (2014) Sensitivity of freshwater pulmonate snail Lymnaea luteola L., to silver nanoparticles. Chemosphere 104:134–140
Almeida EA, Miyamoto S, Bainy ACD, Medeiros MH, Mascio P (2004) Protective effect of phosphor lipid hydroperoxide glutathione peroxidase (PHGPx) against lipid peroxidation in mussels Perna perna exposed to different metals. Mar Pollut Bull 49:386–392
American Public Health Association, American Water Works Association, Water Pollution Control Federation (2005) Standard methods for the examination of water and wastewater, 21st edn. American Publication of Health Association, Washington, DC
Anderson D, Yu TW, Phillips BJ, Schmerzer P (1994) The effect of various antioxidants and other modifying agents on oxygen-radical generated DNA damage inhuman lymphocytes in the comet assay. Mutat Res 307:261–271
Angeletti D, Sebbio C, Carere C, Cimmaruta R, Nascetti G, Pepe G et al (2013) Terrestrial gastropods (Helix spp) as sentinels of primary DNA damage for biomonitoring purposes: a validation study. Environ Mol Mutagen 54(3):204–212
Beers RF Jr, Sizers IW (1952) Spectrophotometric method for measuring the break-down of hydrogen peroxide by catalase. J Biol Chem 195:133–140
Benton MJ, Malott ML, Trybula J, Dean DM, Guttman SI (2002) Genetic effects of mercury contamination on aquatic snail populations: allozyme genotypes and DNA strand breakage. Environ Toxicol Chem 21:584–589
Bradford MM (1976) A rapid and sensitive method for the quantization of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Choi H, Stathatos E, Dionysiou DD (2006) Sol-gel preparation of mesoporous photocatalytic TiO2 films and TiO2/Al2O3 composite membranes for environmental applications. Appl Catal B 63:60–67
Daughton CG (2004) Non-regulated water contaminants: emerging research. Environ Impact Assess Rev 24:711–773
Depledge MH, Aagaard A, Gyorkos P (1995) Assessment of trace metal toxicity using molecular, physiological and behavioural biomarkers. Mar Pollut Bull 31(1–3):19–27
Finney DJ (1971) Probit analysis, 3rd edn. Cambridge University Press, London, p 318
Gottschalk F, Sun TY, Nowack B (2013) Environmental concentrations of engineered nanomaterials review of modeling and analytical studies. Environ Pollut 181:287–300
Jamil K (2001) Bioindicators and biomarkers of environmental pollution and risk assessment. Science, Enfield, NH, pp 45–52
Kaida T, Kobayashi K, Adachi M, Suzuki F (2004) Optical characteristics of titanium oxide interference film and the film laminated with oxides and their applications for cosmetics. J Cosmet Sci 55:219–220
Kovochich M, Xia T, Xu J, Yeh JI, Nel AE (2007) Principles and procedures to assess nanomaterial toxicity. In: Wiesner MR, Bottero JY (eds) Environmental nanotechnology: applications and impacts of nanomaterials. McGraw Hill, New York, pp 205–229
Lee KJ, Nallathamby PD, Browning LM, Osgood CJ, Xu XHN (2007) In vivo imaging of transport and biocompatibility of single silver nanoparticles in early development of zebrafish embryos. ACS Nano 1:133–143
Li S, Wallis LK, Ma H, Diamond SA, Hoff DJ (2014a) Species sensitivity and dependence on exposure conditions impacting the phototoxicity of TiO2 nanoparticles. Environ Toxicol Chem 9999:1–7
Li S, Wallis LK, Ma H, Diamond SA (2014b) Phototoxicity of TiO2 nanoparticles to a freshwater benthic amphipod: are benthic systems at risk? Sci Total Environ 466–467:800–808
Li S, Pan X, Fan ZY, Wallis LK, Chen ZL, Diamond SA (2014c) Comparison of the phototoxicity of TiO2 nanoparticle and graphene-TiO2 nanoparticle composite in Daphnia magna and Oryzias latipes. Chemosphere 112:62–69
Lin J, Zhang H, Chen Z, Zheng Y (2010) Penetration of lipid membranes by gold nanoparticles: Insights into cellular uptake, cytotoxicity, and their relationship. ACS Nano 4(9):5421–5429
Liu K, Lin X, Zhao J (2013) Toxic effects of the interaction of titanium dioxide nanoparticles with chemicals or physical factors. Int J Nanomed 8:2509–2520
Livingstone DR, Lips F, Garcia MP, Pipe RK (1992) Antioxidant enzymes in the digestive gland of the common mussel Mytilus edulis. Mar Biol 112:265–276
Ma H, Wallis LK, Li S, Cañas-Carrell JE, Parra AM, Diamond SA (2014) Toxicity of zinc oxide nanoparticles: Role of particle dissolution and photocatalytic reactive oxygen species (ROS) production. Environ Pollut 193:165–172
Mitchelmore CL, Chipman JK (1998) DNA strand breakage in aquatic organisms and the potential value of the comet assay in environmental monitoring. Mutat Res Fundam Mol Mech Mutagen 399:135–147
Mohamed AH (2011) Sublethal toxicity round up to immunological and molecular aspects of Biomphalaria alexandria to Schistosoma mansoni infection. Ecotoxicol Environ Saf 74:754–760
Moore MN (2006) Do nanoparticles present ecotoxicological risks for the health of the aquatic environment? Environ Sci Technol 32:967–976
Nair V, Turner GE (1984) The thiobarbituric acid test for lipid peroxidation structure of the adduct with malondialdehyde. Lipids 19:84–95
Nigel C, Mitchell ME (2007) Nanoscale: issues and perspectives for the nano century. Wiley, Hoboken
Oberdorster G, Oberdorster E, Oberdorster J (2005) Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect 113:823–839
Organization for Economic Cooperation and Development (1992) Guideline for the testing of chemicals: fish, acute toxicity test. Document p 203
Owens WI, Belcher RV (1965) A colorimetric micro-method for the determination of glutathione. Biochem J 94:705–711
Pavlica M, Klobuèar GIV, Moja N, Erben R, Pape D (2001) Detection of DNA damage in haemocytes of zebra mussel using comet assay. Mutat Res 490:209–214
Rank J (1999) Use of comet assay on the blue mussel, Mytilus edulis, from coastal waters in Denmark. Neoplasma 46:9–10
Regoli F, Hummel H, Amiard-Triquet C, Larroux C, Sukhotin A (1998) Trace metals and variations of antioxidant enzymes in Arctic bivalve populations. Arch Environ Contam Toxicol 35:594–601
Rocco L, Frenzilli G, Zito G, Archimandritis A, Peluso C, Stingo V (2012) Gentotoxic effects in fish induced by pharmacological agents present in the sewage of some Italian water-treatment plants. Environ Toxicol 27:18–25
Roling JA, Baldwin WS (2006) Alterations in hepatic gene expression by trivalent chromium in Fundulus heteroclitus. Mar Environ Res 62:122–127
Sarkar A, Bhagat J, Ingole B, Markad V, Rao DP (2013) Genotoxicity of cadmium chloride in marine gastropod, Nerita chamaeleon using comet assay and alkaline unwinding assay. Environ Toxicol
Singh NP, McCoy MT, Tice RR, Schneider EL (1988) A simple technique for quantization of low levels of DNA damage in individual cells. Exp Cell Res 175:184–191
Torres MA, Testa CP, Gaspari C, Masutti MB, Panitz CMN, Curi-Pedrosa R et al (2002) Oxidative stress in the mussel Mytella guyanensis from polluted mangroves on Santa Catarina Island. Mar Pollut Bull 44:923–932
Valencia A, Kochevar IE (2006) Ultraviolet A induces apoptosis via reactive oxygen species in a model for Smith–Lemli–Opitz syndrome. Free Radic Biol Med 40:641–650
Verma RS, Mehta A, Srivastava N (2007) In vivo chlorpyrifos induced oxidative stress: attenuation by antioxidant vitamins. Pestic Biochem Physiol 88:191–196
Vessey DA, Boyer TD (1984) Differential activation and inhibition of different forms of rat liver glutathione S-transferase by the herbicides 2,4-dichloro phenoxy acetate (2,4-D) and 2,4, Strichloro phenoxy acetate (2,4, S-T). Toxicol Appl Pharmacol 73:492–499
Wallis LK, Diamond SA, Hoff DJ, Al-Abed SR, Li S (2014) Chronic TiO2 nanoparticle exposure to a benthic organism, Hyalella azteca: impact of solar UV radiation and material surface coatings on toxicity. Sci Total Environ 499:356–362
Zhao M, Antunes F, Eaton JW, Brunk UT (2003) Lysosomal enzymes promote mitochondrial oxidant production, cytochrome c release and apoptosis. Eur J Biochem 270:3778–3786
Acknowledgment
The authors their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding this Research group NO (RG -1435-076).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ali, D., Ali, H., Alarifi, S. et al. Impairment of DNA in a Freshwater Gastropod (Lymnea luteola L.) After Exposure to Titanium Dioxide Nanoparticles . Arch Environ Contam Toxicol 68, 543–552 (2015). https://doi.org/10.1007/s00244-015-0132-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-015-0132-0