Abstract
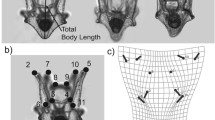
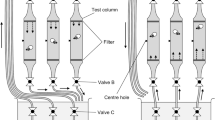
Kinematics of swimming behavior of larval Atlantic cod, aged 12 and 27 days post-hatch (dph) and cultured under three pCO2 conditions (control-370, medium-1800, and high-4200 μatm) from March to May 2010, were extracted from swim path recordings obtained using silhouette video photography. The swim paths were analyzed for swim duration, distance and speed, stop duration, and horizontal and vertical turn angles to determine whether elevated seawater pCO2—at beyond near-future ocean acidification levels—affects the swimming kinematics of Atlantic cod larvae. There were no significant differences in most of the variables tested: the swimming kinematics of Atlantic cod larvae at 12 and 27 dph were highly resilient to extremely elevated pCO2 levels. Nonetheless, cod larvae cultured at the highest pCO2 concentration displayed vertical turn angles that were more restricted (median turn angle, 15°) than larvae in the control (19°) and medium (19°) treatments at 12 dph (but not at 27 dph). Significant reduction in the stop duration of cod larvae from the high treatment (median stop duration, 0.28 s) was also observed compared to the larvae from the control group (0.32 s) at 27 dph (but not at 12 dph). The functional and ecological significance of these subtle differences are unclear and, therefore, require further investigation in order to determine whether they are ecologically relevant or spurious.






Similar content being viewed by others
References
Bates NR, Mathis JT (2009) The Arctic Ocean marine carbon cycle: evaluation of air-sea CO2 exchanges, ocean acidification impacts and potential feedbacks. Biogeosciences 6:2433–2459. doi:10.5194/bg-6-2433-2009
Bates NR, Mathis JT, Cooper LW (2009) Ocean acidification and biologically induced seasonality of carbonate mineral saturation states in the western Arctic Ocean. J Geophys Res 114:C11007. doi:10.1029/2008JC004862
Batschelet E (1981) Circular statistics in biology. Academic Press, New York
Browman HI, O’Brien WJ (1992) The ontogeny of search behaviour in the white crappie, Pomoxis annularis. Environ Biol Fishes 34:181–195. doi:10.1007/BF00002393
Browman HI, Novales-Flamarique I, Hawryshyn CW (1994) Ultraviolet photoreception contributes to prey search behaviour in two species of zooplanktivorous fishes. J Exp Biol 186:187–198
Browman HI, St-Pierre JF, Skiftesvik AB, Racca RG (2003) Behaviour of Atlantic cod (Gadus morhua) larvae: an attempt to link maternal condition with larval quality. In: Browman HI, Skiftesvik AB (eds) The Big Fish Bang. Proceedings of the 26th Annual Larval Fish Conference. Institute of Marine Research, Bergen, Norway. pp 71–95
Coughlin DJ, Strickler JR, Sanderson B (1992) Swimming and search behaviour in clownfish, Amphiprion perideraion, larvae. Anim Behav 44:427–440. doi:10.1016/0003-3472(92)90053-C
Cripps IL, Munday PL, McCormick MI (2011) Ocean acidification affects prey detection by a predatory reef fish. PLoS ONE 6(7):e22736. doi:10.1371/journal.pone.0022736
Dixson DL, Munday PL, Jones GP (2010) Ocean acidification disrupts the innate ability of fish to detect predator olfactory cues. Ecol Lett 13:68–75. doi:10.1111/j.1461-0248.2009.01400.x
Domenici P (2001) The scaling of locomotor performance in predator-prey encounters: from fish to killer whales. Comp Biochem Physiol Part A 131:169–182. doi:10.1016/S1095-6433(01)00465-2
Domenici P, Allan B, McCormick MI, Munday PL (2012) Elevated carbon dioxide affects behavioural lateralization in a coral reef fish. Biol Lett 8(1):78–81. doi:10.1098/rsbl.2011.0591
Edmunds PJ (2011) Zooplanktivory ameliorates the effects of ocean acidification on the reef coral Porites spp. Limnol Oceanogr 56(6):2402–2410. doi:10.4319/lo.2011.56.6.2402
Ellertsen B, Fossum P, Solemdal P, Sundby S, Tilseth S (1984) A case study on the distribution of cod larvae and availability of prey organisms in relation to physical processes in Lofoten. Flødevigen rapportser 1:453–477
Ellertsen B, Fossum P, Solemdal P, Sundby S, Tilseth S (1987) The effect of biological and physical factors on the survival of Arcto-Norwegian cod and the influence on recruitment variability. In: Loeng H (ed) The effect of oceanographic conditions on distribution and population dynamics of commercial fish stocks in the Barents Sea. Proceedings of the third Soviet-Norwegian Symposium, Murmansk, pp 101–126
Fabry VJ, McClintock JB, Mathis JT, Grebmeier JM (2009) Ocean acidification at high latitudes: The bellwether. Oceanography 22(4):160–171. http://dx.doi.org/10.5670/oceanog.2009.105
Ferrari MCO, Manassa RP, Dixson DL, Munday PL, McCormick MI, Meekan MG, Sih A, Chivers DP (2012) Effects of ocean acidification on learning in coral reef fishes. PLoS ONE 7(2):e31478. doi:10.1371/journal.pone.0031478
Findlay HS, Tyrrell T, Bellerby RGJ, Merico A, Skjelvan I (2008) Carbon and nutrient mixed layer dynamics in the Norwegian Sea. Biogeosciences 5:1395–1410. doi:10.5194/bg-5-1395-2008
Frommel AY, Maneja RH, Lowe D, Malzahn AM, Geffen AJ, Folkvord A, Piatkowski U, Reusch T, Clemmesen C (2012) Severe tissue damage in Atlantic cod larvae under increasing ocean acidification. Nat Clim Chang 2:42–46. doi:10.1038/nclimate1324
Galbraith PS, Browman HI, Racca RG, Skiftesvik AB, Saint-Pierre JF (2004) Effect of turbulence on the energetics of foraging in Atlantic cod Gadus morhua larvae. Mar Ecol Prog Ser 281:241–257. doi:10.3354/meps281241
Gattuso J-P, Lavigne H (2009) Technical Note: approaches and software tools to investigate the impact of ocean acidification. Biogeosciences 6:2121–2133. doi:10.5194/bg-6-2121-2009
Gislefoss JS, Nydal R, Slagstad D, Sonninen E, Holme K (1998) Carbon time series in the Norwegian Sea. Deep Sea Res I 45:433–460. http://dx.doi.org/10.1016/S0967-0637(97)00093-9
Houde ED, Zastrow CE (1993) Ecosystem- and taxon-specific dynamic and energetics properties of larval fish assemblages. Bull Mar Sci 53(2):290–335
Huelsenbeck M (2010) The effects of elevated carbon dioxide concentrations for the larval behaviour of white seabass. Atractoscion nobilis. Master’s thesis. Scripps Institution of Oceanography, UCSD, p 31
Hunt von Herbing I, Gallager SM, Halteman W (2001) Metabolic costs of pursuit and attack in early larval Atlantic cod. Mar Ecol Prog Ser 216:201–212. doi:10.3354/meps216201
IPCC (2007) Climate Change 2007 The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL (eds). Cambridge University Press, Cambridge, UK and New York NY, USA, pp 996
Kristiansen T, Drinkwater KF, Lough RG, Sundby S (2011) Recruitment variability in North Atlantic cod and match-mismatch dynamics. PLoS ONE 6(3):e17456. doi:10.1371/journal.pone.0017456
Lewis E, Wallace D (1998) Program developed for CO2 systems calculations, ORNL/CDIAC-105. Carbon Dioxide Information Analysis Centre, Oak Ridge National Laboratory, US Department of Energy, Oak Ridge
Melzner F, Göbel S, Langenbuch M, Gutowska MA, Pörtner H-O, Lucassen M (2009) Swimming performance in Atlantic cod (Gadus morhua) following long-term (4–12 months) acclimation to elevated seawater pCO2. Aqua Toxicol 92:30–37. http://dx.doi.org/10.1016/j.aquatox.2008.12.011
Melzner F, Stange P, Trübenbach K, Thomsen J, Casties I, Panknin U, Gorb SN, Gutowska MA (2011) Food supply and seawater pCO2 impact calcification and internal shell dissolution in the blue mussel Mytilus edulis. PLoS ONE 6(9):e24223. doi:10.1371/journal.pone.0024223
Munday PL, Crawley NE, Nilsson GE (2009a) Interacting effects of elevated temperature and ocean acidification on the aerobic performance of coral reef fishes. Mar Ecol Prog Ser 388:235–242. doi:10.3354/meps08137
Munday PL, Dixson DL, Donelson JM, Jones GP, Pratchett MS, Devitsina GV, Døving KB (2009b) Ocean acidification impairs olfactory discrimination and homing ability of a marine fish. Proceedings of the National Academy of Sciences of the Unites States of America 106 (6):1848–1852. doi:10.1073/pnas.0809996106
Munday PL, Dixson DL, McCormick MI, Meekan M, Ferrari MCO, Chivers DP (2010) Replenishment of fish populations is threatened by Ocean acidification. Proc Nat Acad Sci USA 107(29):12930–12934. doi:10.1073/pnas.1004519107
Nilsson GE, Dixson DL, Domenici P, McCormick MI, Sørensen C, Watson S-A, Munday PL (2012) Near-future carbon dioxide levels alter fish behaviour by interfering with neurotransmitter function. Nat Clim Chang 2:201–204. doi:10.1038/nclimate1352
Nowicki JP, Miller GM, Munday PL (2012) Interactive effects of elevated temperature and CO2 on foraging behavior of juvenile coral reef fish. J Exp Mar Biol Ecol 412:46–51. http://dx.doi.org/10.1016/j.jembe.2011.10.020
O’Brien WJ, Evans BI, Browman HI (1989) Flexible search tactics and efficient foraging in saltatory searching animals. Oecologia 80:100–110. doi:10.1007/BF00789938
Pörtner HO, Langenbuch M, Michaelidis B (2005) Synergistic effects of temperature extremes, hypoxia, and increases in CO2 on marine animals: From earth history to global change. J Geophys Res 110: C09S10, doi:10.1029/2004JC002561
Puvanendran V, Leader LL, Brown JA (2002) Foraging behaviour of Atlantic cod (Gadus morhua) larvae in relation to prey concentration. Can J Zool 80:689–699. doi:10.1139/Z02-045
Ruzicka JJ (2004) Integrating bioenergetics and foraging behaviour: The physiological ecology of larval cod (Gadus morhua). PhD dissertation. Massachusetts Institute of Technology and Woods Hole Oceanographic Institution, USA, p 194
Ruzicka JJ, Gallager SM (2006) The saltatory search behaviour of larval cod (Gadus morhua). Deep Sea Res II 53:2735–2757. http://dx.doi.org/10.1016/j.dsr2.2006.09.003
Seljeset O, Vollset K, Folkvord A, Geffen AJ (2010) The role of prey concentration and size range in the growth and survival of larval cod. Marine Biol Res 6:251–262. doi:10.1080/17451000903150355
Simpson SD, Munday PL, Wittenrich ML, Manassa R, Dixson DL, Gagliano M, Yan HY (2011) Ocean acidification erodes crucial auditory behaviour in a marine fish. Biol Lett 7(6):917–920. doi:10.1098/rsbl.2011.0293
Skajaa K, Browman HI (2007) The escape response of food-deprived cod larvae (Gadus morhua L.). J Exp Mar Biol Ecol 353:135–144. doi:10.1016/j.jembe.2007.01.014
Skiftesvik AB, Browman HI, St-Pierre JF (2003) Life in green water: the effect of microalgae on the behaviour of Atlantic cod (Gadus morhua) larvae. In: Browman HI, Skiftesvik AB (eds) The Big Fish Bang. Proceedings of the 26th Annual Larval Fish Conference. Institute of Marine Research, Bergen, Norway. pp 97–103
Steinacher M, Joos F, Frölicher TL, Plattner GK, Doney SC (2009) Imminent ocean acidification in the Arctic projected with the NCAR global coupled carbon cycle-climate model. Biogeosciences 6:515–533. doi:10.5194/bg-6-515-2009
Suthers IM, Sundby S (1993) Dispersal and growth of pelagic juvenile Arcto-Norwegian cod (Gadus morhua), inferred from otolith microstructure and water temperature. ICES J Mar Sci 50:261–270. doi:10.1006/jmsc.1993.1028
Tilseth S (1984) The distribution of cod larvae and prey organisms in the Lofoten area related to critical prey concentrations. In: Godø OR, Tilseth S (eds) Reproduction and recruitment of Arctic cod. Proceedings of the Soviet-Norwegian Symposium, Leningrad, pp 36–71
Videler JJ (1993) Fish Swimming. Fish and Fisheries Series 10. Chapman and Hall, London, p 269
Vollset KW, Folkvord A, Browman HI (2011) Foraging behaviour of larval cod (Gadus morhua) at low light intensities. Mar Biol 158:1125–1133. doi:10.1007/s00227-011-1635-5
Webb PB (1981) Responses of northern anchovy, Engaulis mordax, larvae to predation by a biting planktivore Amphiprion percula. Fish Bull 79(4):727–735
Acknowledgments
Funding support was provided through the European Marie Curie Initial Training Network “Calcification by Marine Organisms” (CalMarO) and the European Community’s Seventh Framework Programme (FP7/2007-2013) “European Project on Ocean Acidification” (EPOCA, grant agreement N211384). The study was also supported by the project “Biological Impacts of Ocean ACIDification” (BIOACID), funded by the German Ministry for Education and Research (BMBF), and by the Norwegian Institute of Marine Research (“Fine-scale behavioral interactions in the plankton” and “Biological effects of ocean acidification” projects to HIB). The experiments were conducted at the Norwegian National Mesocosm Centre, Espegrend, in cooperation with the Department of Biology, University of Bergen and at the Institute of Marine Research’s Austevoll Research Station.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Dupont.
Rights and permissions
About this article
Cite this article
Maneja, R.H., Frommel, A.Y., Browman, H.I. et al. The swimming kinematics of larval Atlantic cod, Gadus morhua L., are resilient to elevated seawater pCO2 . Mar Biol 160, 1963–1972 (2013). https://doi.org/10.1007/s00227-012-2054-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-012-2054-y