Abstract
Satellite transmitters were attached to 25 reproductively active and four inactive adult male loggerhead sea turtles (86.6–107.0 cm SCLmin) captured from the Port Canaveral, FL, USA shipping channel to assess horizontal and vertical distributions. During the breeding period, male loggerheads aggregated (44% of 755 turtle days) in a 117.6 km2 core area that encompassed the shipping channel. Median dive duration during the breeding period was 27 min (IQR = 15–42 min) and males spent 4% (IQR = 3–5%) of the time at the surface, with significantly shorter dives associated with reproductively active males. Migrant and resident males dispersed concurrently, with residents shifting > 30 km east across the continental shelf over a more protracted departure schedule than migrants. Dive duration and time spent at the surface increased through the fall. Cluster analysis revealed the strongest association for dive duration with sea state during and after the breeding period, with significantly longer dives during more turbulent conditions. In contrast, univariate associations with surface interval duration were not elucidated.
Similar content being viewed by others
Introduction
Environmental requirements for successful nesting and hatchling emergence are well documented for sea turtles (Miller 1996); however, our understanding of the requirements for successful courtship and mating are less well defined. Sea turtle courtship and mating data include in-water (Booth and Peters 1972; Rostal et al. 1998; Wibbels et al. 1990; Schofield et al. 2006) and aerial observations of mounted pairs at the water surface (Frick et al. 2000; Godley et al. 2002). Adult male sea turtles expend considerable effort and energy to locate and breed with adult females (Jessop et al. 2004; Schofield et al. 2006); thus, mating success may not be temporally constant, particularly if warmer air (and subsequently incubation) temperatures produce fewer males in the future (Hawkes et al. 2007; Coyne and Landry 2007) given temperature-dependent sex determination in sea turtles (Standora and Spotilla 1985). For most sea turtle species, the long standing paradigm is that mating occurs near nesting beaches (Lazell 1980) and peaks a month prior to first oviposition (Owens 1980; Miller 1996), after which adult males disperse to seasonal foraging grounds as females become unreceptive and begin nesting (Booth and Peters 1972).
Tagging and satellite telemetry studies with adult male sea turtles corroborate the suggestion of reproductive migrations (Balazs 1983; Plotkin et al. 1996; James et al. 2005), but also reveal that some males are year-round residents (Shaver et al. 2005; van Dam et al. 2008; Schofield et al. 2009, 2010a, b). These studies also indicate that adult males often breed annually and display high affinity for both seasonal foraging and breeding grounds (Balazs 1983; James et al. 2005; van Dam et al. 2008), with the latter often characterized by large seasonal aggregations of adults. As such, adult male sea turtles may be quite vulnerable to anthropogenic impacts such as fishing gear entanglement (Plotkin et al. 1996; Shaver et al. 2005) when located en masse on seasonal breeding grounds, which often occur close to shore in shallow waters (Godley et al. 2002). Despite such concerns, the distributional patterns of adult male sea turtles at even large seasonal breeding grounds remain largely unknown. For example, hundreds of adult male loggerheads have been captured from or observed at Heron Atoll in Australia (Wibbels et al. 1990), Laganas Bay, Zakynthos Island, Greece (Margaritoulis 2005; Schofield et al. 2006), and the Port Canaveral, FL, USA shipping channel (Henwood 1987), yet seasonal movements are only reported for 13 adult males in Greece (Schofield et al. 2009, 2010a, b; Hays et al. 2010).
The coastal waters adjacent to Port Canaveral, FL have been extensively sampled for sea turtles (Henwood 1987; Dickerson et al. 1995; Schmid 1995), yet distribution data are sparse. Henwood (1987) noted peak annual captures of adult male loggerheads between February and April, with reduced collection of adult males in May concurrent with an influx of adult females. Adult males tagged in the shipping channel have been recaptured there, but data for adult males tagged in the channel and recaptured elsewhere are not reported (Henwood 1987). Three adult males acoustically tracked for 2 days during a breeding period remained localized to the shipping channel (Standora et al. 1993), similar to three radio-tracked adult males that remained within 10 km of shore for up to 6 days in a nonbreeding period (Kemmerer et al. 1983).
Given that Port Canaveral is a major commercial seaport located approximately 40 km north of the second largest loggerhead rookery in the world (NMFS and USFWS 2008), available data were deemed insufficient to make broad conclusions about the seasonal distribution of male loggerhead sea turtles at this important location and the current study was initiated. Here, we present geographic data collected using satellite telemetry to test the hypothesis that the distribution of adult male loggerheads near the Port Canaveral shipping channel is similar during and after breeding. The hypotheses of no differences in dive and surface interval times between reproductively active and inactive males during and after the breeding period are also evaluated.
Materials and methods
Sea turtle collection and processing
Adult male loggerheads were captured by trawling (15 min, bottom time) from the R/V Georgia Bulldog in the shipping channel at Port Canaveral, FL, USA (28°23′N, −80°32′W) between channel buoy pairs “1–2” and “9–10”. Trawling was completed during three independent 5-day research cruises in 2006 (17–21 April) and 2007 (2–6 April, 25–29 April). Trawling utilized paired standardized National Marine Fisheries Service (NMFS) turtle nets (19.9 m, head rope). Turtles were removed from nets and evaluated for general health status, injuries and existing tags before being assigned a unique project identification number.
Individuals were field identified as male given extension of the tail posterior of the carapace, and sex was confirmed via laparoscopy (Blanvillain et al. 2008). Minimum straight-line carapace length (SCLmin, cm) was measured with tree calipers and body mass (kg) recorded using a spring scale. Blood samples (<15 ml) were collected from the dorsal cervical sinus (Owens and Ruiz 1980) using a 21-gauge, 3.5-cm needle and preserved at sea for subsequent analysis of testosterone concentration and mitochondrial DNA (mtDNA) haplotype in a shore laboratory. Serum testosterone concentration was quantified using radioimmunoassay described in Blanvillain et al. (2008). Haplotype designations (Bowen et al. 2004) were assigned after sequencing a 378 base pair (bp) fragment of the mtDNA control region (described in Roberts et al. 2005) and compared to regional nesting beach data (Encalada et al. 1998; Bowen et al. 2004).
Data collection
Satellite transmitters (ST-20, Model A2020; Telonics, Inc., Mesa, Arizona) were attached to the second vertebral scute (Polovina et al. 2000) after barnacles and other organisms were removed with a chisel and the anterior carapace was sanded, washed with betadine™ and dried with acetone. A roll of 1.0 cm diameter Sonic Weld™ (Ed Greene & Company; Sparta, Tennessee) placed around the bottom of the transmitter formed a well that was filled with a liquid epoxy resin (Fast Foil™, Power Fasteners, Inc.; New Rochelle, NY). Transmitters with the antenna oriented anteriorly were pressed onto the carapace and additional Fast Foil™ was applied. Male loggerheads were released 3.7 ± 1.7 km (Mean ± SD) from the shipping channel; however, we did not consider this an unreasonable displacement given the navigational capabilities of adult male loggerheads (Schofield et al. 2009). Twelve males were released the same day (7.2 ± 2.0 h after capture) and 17 males were held overnight and released 22.8 ± 2.8 h after capture. Telemetry data were collected until the transmitters stopped providing data.
Transmitter duty cycle was set to continuous on with a 45-s repetition rate at 401.630 to 401.656 MHz; however, a saltwater switch (with 4 h failsafe duration) was included to conserve battery life while turtles were submerged. In addition to geographic position, we collected data on ambient temperature (°C) and three dive cycle metrics corresponding to the number of dives, mean dive duration (s) and the percent of time submerged during 4, 6-h data bins each day (1900–0059 h EST; 0100–0659 h; 0700–1259 h; 1300–1859 h).
Data processing
Telemetry data processed and distributed by CLS America (Largo, MD) were archived and associated with a water depth and distance from shore via the satellite tracking and analysis tool (STAT; Coyne and Godley 2005). Locations with elevations > 1 m above sea level (8.3% of original location data) were removed (Mansfield et al. 2009; Schofield et al. 2010a, b). An initial daily “best” location for each turtle was selected as the first occurrence of the most accurate location class according to the CLS America hierarchy (3 > 2 > 1 > 0 > A > B > Z). Selected locations were then simultaneously subjected to speed (3 km h−1) and inner angle (5°) filters created by the authors (Supplement 1). Locations flagged by these filters were removed and the “next best” location systematically substituted until filter criteria were met. If substitute locations were not available or if no substitutions met filter criteria, no location data for that turtle for that day (i.e., a turtle day) were retained for analyses. Post-filtering, 96.6 ± 13.5% (Mean ± SD) of turtle days of data for individual loggerheads were retained.
Transmitter sensor data were also filtered prior to analysis. Temperature records >40 and <5°C were removed (<1% of original temperature data) given a high improbability of occurrence (National Data Buoy Center; http://www.ndbc.noaa.gov/station_page.php?station=41009). All data for binned dive metrics (number of dives, mean dive duration, submergence) were removed when mean dive duration (s) multiplied by the number of dives exceeded the total possible seconds in 6 h. A single observation for each turtle and unique data collection bin was selected to standardize observation weights. When replicate observations for a dive metric occurred during a data bin, the mode was selected to provide a single observation per unique bin. When no mode was observed, the mean was computed if the maximum difference among replicate values was <5% relative (dive duration) or <5% absolute (surface interval); otherwise all replicate observations were removed. Total bin retention (mean ± SD) among individual turtles for dive duration and surface interval data was 73 ± 15% and 85 ± 8%, respectively.
Data analysis
Adult male loggerheads that remained between 28°N and 29°N were classified as residents.
Location data were split into breeding and post-breeding periods which were separated by distinct movements as noted for adult male sea turtles in previous studies (Plotkin et al. 1996; Shaver et al. 2005; Schofield et al. 2009). For resident males, the end of the breeding period was marked by a directed easterly shift ≥20 km, whereas after the breeding period migrant males dispersed rapidly to the north or south. Two adult male loggerheads detected for 7 and 46 d did not outwardly disperse away from Port Canaveral and were classified as “unknown”. Location data for all satellite-tagged adult males were analyzed during the breeding period; however, spatial distribution was only analyzed for resident males after the breeding period.
Minimum convex polygons (MCPs) were created (Geospatial Modelling Environment; www.spatialecology.com) around all filtered locations for (a) breeding and (b) post-breeding data collection periods and their respective areas (km2) were computed. Core area use within the respective MCPs was analyzed using Hot Spot analysis (ArcGIS ArcInfo Build 2800; ESRI, Redlands, CA), which compared the count of locations within 1 min latitude × 1 min longitude grids to their “neighbors” to assess clustering of high or low values. All 1 min grids (features) had at least eight neighbors as a result of the Generate Spatial Weights Matrix script reaching out further than minimum distance bands (5,500 and 3,500–14,000 m before and after the breeding period, respectively) denoted by Z-scores in a precursor step. During the breeding period, resident and migrant distributions overlapped for significant (P ≤ 0.01) grids; thus, data were pooled and analyzed in aggregate. MCPs were created around statistically significant grids and their subsequent area (km2) calculated.
Cluster analysis was performed in Minitab 15® to assess the phenology of adult male loggerhead dispersal (Julian date) away from Port Canaveral with respect to seven variables: reproductive status (0 = active, 1 = inactive); release group (1 = 2–6 April; 2 = 16–20 April; 3 = 25–29 April); SCLmin (cm); body mass (kg); haplotype (0 = no sample; 1 = CC-A01; 2 = CC-A02; 3 = other); displacement distance (km) between the shipping channel (28.385°N, −80.542°W) and median post-dispersal location for each male loggerhead; and serum testosterone level (ng ml−1) at the time of initial turtle capture. All resulting single variable cluster relationships were analyzed statistically using linear regression (normally distributed residuals) or Kruskal–Wallis (K–W) analysis of variance by ranks (non-normally distributed residuals), with K–W groupings defined ad hoc to distribute samples as evenly as possible.
Cluster analysis was also used to evaluate associations between mean dive duration (s) and percent of time spent at the surface during 6-h data bins during and after the breeding period with 18 variables: loggerhead group (1 = reproductively active residents, 2 = reproductively inactive residents, 3 = migrants, 4 = unknown final disposition); turtle size (SCLmin, cm); body mass (kg); serum testosterone (ng ml−1); haplotype (0 = CCA01, 1 = CC-A02, 2 = other, 3 = unknown); Julian day; sequential observation day; time of day (1 = 1900–0059 h EST, 2 = 0100–0659 h, 3 = 0700–1259 h, 4 = 1300–1859 h); longitude; latitude; water depth (m); distance (km) from shore; rate of travel (km d−1) between successive locations; daily mean and standard deviation in ambient temperature (°C); daily mean water temperature (°C); daily mean air temperature (°C); daily mean barometric pressure (mb); daily mean wave height (m); and daily mean wind speed (m s−1). Meteorological and hydrographic metrics were computed from measurements at the Canaveral offshore buoy (41009, National Data Buoy Center).
Body length residuals were normally distributed; thus, resident versus migrant size distribution was analyzed (α = 0.05 for all tests) using a One-Way Analysis of Variance in Minitab 15® (Minitab, Inc., State College, PA). Dive and surface duration residuals were not normally distributed; thus comparisons between reproductively active and inactive adult males were performed using Kruskal–Wallis analysis of variance by ranks in Minitab 15®. The ratio of CC-A01 to CC-A02 between resident and migrant males was compared statistically with a two-tailed Fisher’s exact test in Vassar Stats (http://faculty.vassar.edu/lowry/fisher.html).
Results
General
Twenty-nine adult male loggerheads collected by trawling in the Port Canaveral, FL shipping channel between 17–21 April 2006 (n = 9), 2–6 April 2007 (n = 10) and 25–29 April 2007 (n = 10) were monitored for 51.0 ± 49.7 d (mean ± SD) at this study location (Table 1). Size distribution was bimodal (Fig. 1), and residents (86.6–93.2 cm SCLmin, n = 11) were significantly smaller (F 1 = 4.11, P = 0.028) than migrants (86.8–107 cm SCLmin, n = 16). Initial serum testosterone levels ranged from 1.3 to 188.4 ng ml−1 (Table 1) and Blanvillain et al. (2008) determined that all but four of 27 adult males (all residents) were reproductively active. CC-A01 and CC-A02 accounted for 90% of observed haplotypes (Table 1) and occurred with different proportions among nine residents (CC-A01 = 0.30; CC-A02 = 0.60) and 15 migrants (CC-A01 = 0.69; CC-A02 = 0.25), but differences were not statistically significant (P = 0.092).
The relationship between body length (SCLmin, cm) versus body mass (kg) among four reproductively inactive adult male loggerhead sea turtles (open diamond, all residents) and 25 reproductively active adult male loggerhead sea turtles consisting of seven residents (filled diamond), 16 migrants (gray triangle) and two males of unknown distribution (plus symbol)
Similar quantities of data (Table 1) were collected during and after the breeding period, but with different proportions of data collected during both periods with respect to reproductive activity. Twenty-five active adult male loggerheads comprised 91% (n = 686 of 755 turtle days, td) of data collected during the breeding period, whereas four reproductively inactive adult male loggerheads represented 44% (n = 408 of 723 td) of the data analyzed after the breeding period, predominantly associated with males 64542 and 64545 (Table 1). Location class quality during the breeding period was skewed (63%) toward less reliable “A” and “B” detections, which were analyzed half as frequently (33%) after the breeding period due to the top three location classes (3, 2 and 1) being twice as available (46% vs. 21%; Supplement 2).
Spatial distribution
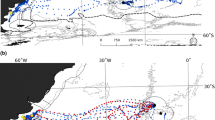
Filtered daily locations for adult male loggerheads during the breeding period were distributed within a total MCP area of 4,019.7 km2 that extended offshore to water depths < 60 m as well as inshore in the Indian River and Banana River lagoon complex (Fig. 2). During the breeding period, 44% (n = 335 td) of adult male loggerhead locations occurred in a core area that measured 117.6 km2 (3% of MCP and 15% of 304 breeding period grids). During the breeding period, adult male loggerheads were located 12.1 ± 11.7 km (mean ± SD) from the shipping channel, and were detected within 5 km of the channel 36% (n = 268 of 755 td) of the time. During the breeding period, adult male loggerheads occurred 6.1 ± 6.2 km (n = 755 td) from shore in waters 7.4 ± 7.3 m deep (n = 755 td) at temperatures (24.4 ± 1.3°C; n = 5,899 observations).
Spatial distribution of adult male loggerheads in the vicinity of Cape Canaveral, FL, USA during (n = 755 td) and after (n = 723 td) the breeding period. Overall, MCP area for the breeding period is denoted by a black dashed line, with the shaded area labeled “1” denoting the core area for this period centered on the channel (solid line). Overall, MCP area after the breeding period is denoted by a gray dashed line. After the breeding period, reproductively active resident males utilized an inner and outer core area (2) while reproductively inactive males utilized a single outer core area (3). The solid line delineates the 100 m depth contour, NDBC buoys 41009 and 4113 are labeled accordingly and two asterisks (*) denote points of access to the Indian River Lagoon complex
Median dispersal date for residents and migrants was 17 May; however, migrants dispersed over a more consolidated (7 May–1 June) schedule than residents (8 April–21 June; Fig. 3). Cluster analysis revealed only a weak association (64% similarity) with all variables combined except for reproductive status (active vs. inactive) or haplotype. Migrants departed 8–47 d (median = 31 d, IQR = 21–36 d) after tag and release, comparable to residents (median = 24 d; IQR = 12–46 d). Half of all migrants were captured between 25 and 29 April and represented 8 of 10 adult male loggerheads captured during that sampling period.
After the breeding period, resident adult male loggerheads were distributed within a total MCP area of 12,206.8 km2 that extended seaward of the 100-m depth contour, but continued to include the inshore waters of the Indian River and Banana River lagoon complex (Fig. 2). Within this MCP, two core areas were identified for formerly reproductively active males. The first core area (7.5 km2) was situated adjacent to and seaward of the breeding period core area; however, after the breeding period adult male loggerheads were only infrequently (n = 21 of 723 td) detected (all prior to 6 August) within 10 km of the channel. A second core area (58.9 km2) situated 35 km from shore was identified for the formerly reproductively active resident males. Formerly inactive adult male loggerheads shared a single core area (511.4 km2) located 55 km from shore. Collectively, these three core areas accounted for 21% (n = 112 of 531) of total grids and 26% (n = 187 of 723) of turtle days. After the breeding period, adult male loggerheads were distributed (mean ± SD) 32.8 ± 12.7 km (n = 709 observations) from shore in waters 42.7 ± 43.9 m deep (n = 720 observations) at temperatures 24.8 ± 2.8°C (n = 4,531 observations).
Dive and surfacing behavior
Reproductively active males made significantly shorter dives (H 1 = 63.19, P < 0.001) during the breeding period (Fig. 4), whereas formerly active resident males made significantly longer dives (H 1 = 15.81, P < 0.001) after the breeding period (Fig. 5a). Median dive duration for all adult male loggerhead sea turtles was 27 min (IQR = 15–42 min; n = 1,638 bins) during the breeding period and 26 min (IQR = 13–45 min; n = 1,761 bins) after the breeding period. Both during (n = 1,446 bins) and after the breeding period (n = 1,512 bins), cluster analysis revealed the strongest association for dive duration (59 and 63% similarity, respectively) with wind speed and wave height which were auto-correlated with each other (84–92% similarity). During both observation periods, dive duration was significantly longer (H 1 = 72.49–76.76, P < 0.001) at wind speeds > 6 m s−1 (median = 33 min (IQR = 20–45 min) during the breeding period, 40 min (IQR = 21–62 min) after the breeding period) than at slower wind speeds (median = 23–24 min (IQR = 12–41 min) during and after the breeding period, respectively).
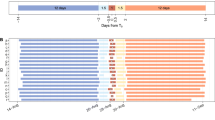
Median (diamond) dive duration and the percent of time spent at the surface during the breeding period in near-shore waters in the vicinity of Cape Canaveral, FL, USA. The bar denotes the inter-quartile range and error bars denote the 5th and 95th percentiles. The number of data bins analyzed for each group is displayed above the 95th percentile
Median (diamond) dive duration (a) and the percent of time spent at the surface (b) by formerly reproductively active (a) and inactive (i) residents after the breeding period near Port Canaveral, FL, USA. Inter-quartile range denoted by bar and 5th and 95th percentiles denoted by error bars. Monthly observations (mean ± SD) for formerly reproductively active and inactive residents were 89 ± 79 and 131 ± 60 bins, respectively
During the breeding period, migrant males spent significantly (H 1 = 43.85, P < 0.001) less time at the surface (median = 3.8%; IQR = 2.9–4.9%; n = 1,002 bins) than other groups. After the breeding period, significantly (H 1 = 75.08, P < 0.001) more time was spent at the surface by formerly inactive males (median = 8.4%; IQR = 6.0–10.9%; n = 1,063 bins) than formerly active males (median = 6.7%; IQR = 4.8–9.3%; n = 774 bins). Median surface interval (n = 1,837 bins for both observation periods) for adult males was 4.0% (IQR = 3.0–5.2%) and 7.7% (IQR = 5.4–10.3%) during and after the breeding period, respectively (Figs. 4, 5b). Cluster analysis revealed no univariate associations with surface interval duration, and only revealed weak associations (52–56% similarity) with all variables combined except for time of day both during (n = 1,642 bins) and after (n = 1,548 bins) the breeding period. Ambient temperatures (Fig. 6) suggested that resident male loggerheads spent the majority of the time submerged below a thermocline depth through September (2006) and October (2007).
Monthly (mean ± 95% CI) transmitter temperatures for reproductively active (dark bar) and inactive (light bar) adult male loggerheads relative to air (square) and water (triangle) temperatures recorded at the Cape Canaveral, Florida offshore data buoy. Monthly transmitter and buoy readings (mean ± SD) were 226 ± 181 and 1,454 ± 42, respectively
Discussion
Despite their resident nature, adult male loggerhead sea turtles exhibited distinctly different distributional patterns with respect to the shipping channel during and after the breeding period. During the breeding period, resident and migrant adult male loggerheads co-occurred close to shore and in an area immediately southwest of a large sandy cape that distinguishes this particular coastline, with the core area also centered on the channel where they were captured. However, after the breeding period resident males shifted offshore to the middle and outer continental shelf and only infrequently occurred near the shipping channel, suggesting that historical capture of adult male loggerheads in the channel during nonbreeding periods (Kemmerer et al. 1983; Henwood 1987; Dickerson et al. 1995) represent low probability events. After the breeding period, adult male loggerheads were distributed over an area three times as large as was utilized during the breeding period, with limited spatial overlap among individuals. A more nomadic nature of resident adult male sea turtles after breeding is also reported for other sea turtle species (Shaver et al. 2005; van Dam et al. 2008; Schofield et al. 2010a, b), and likely reflects a combination of shorter migration distances as well as greater spatial flexibility associated with foraging compared to breeding en masse during a finite window of opportunity.
During the breeding period, adult male loggerheads in the current study were much more widely distributed than during the breeding period at Zakythos Island, which may in part be explained by geomorphological differences between study locations. At Zakynthos Island, mating occurred within the 10 km × 10 km cove of Laganas Bay on the south side of the island associated with a steep beach profile; thus, shallow water depths which are thought to be preferred for breeding (Godley et al. 2002) were spatially restricted. In contrast, the current study was conducted along a relatively uniform stretch of coastline with a shallower beach profile and subsequently adult male loggerheads were more widely distributed. Aerial surveys of mounted loggerhead pairs elsewhere along the US East Coast also confirm wide distribution with respect to distance from shore and water depth (Frick et al. 2000; Wibbels pers comm, University of Alabama, 1300 University Boulevard, Birmingham, AL, 35294, twibbels@uab.edu; Epperly pers comm, NMFS Southeast Fisheries Science Center, 75 Virginia Beach Drive, Miami, FL 33149, Sheryan.Epperly@noaa.gov).
The shallow and near-shore distribution of adult male loggerheads during the breeding period appeared to extend to the Indian and Banana River lagoon complex where adult loggerheads are collected, albeit infrequently, year-round (Wibbels et al. 1987; Witherington and Ehrhart 1989). We believe that loggerheads transited between the coastal ocean and these lagoons via Port Canaveral and a manmade lock at the western boundary of the Port constructed by the US Army Corps of Engineers in 1965. Loggerhead detections in the lagoons radiated outward from the lock, further suggesting that access was through the lock and not through Sebastian Inlet, a natural inlet located 50 km to the south where only one (64546) of our tagged loggerheads was ever detected. The main channel of the Port between the ocean and the Banana River is roughly 6 km long; thus, it is conceivable that loggerheads could transit this channel without being detected by orbiting (and thus only episodically overhead) satellites given that the lock is opened several times daily. An expansive network of submerged receivers that continuously monitor for acoustically tagged animals at selected locations in the Indian River Lagoon system validates occasional sea turtle transit through the lock (Provancha and Reyier pers comm. Innovative Health Applications LLC, Kennedy Space Center, FL; jane.a.provancha@nasa.gov, eric.a.reyier@nasa.gov); however, loggerheads in our study were not equipped with acoustic transmitters that would have enabled them to be detected within that receiver array which now spans the Port from the ocean through the lock. As such, given the noncontinuous monitoring nature of satellite telemetry, we recommend tandem deployment of acoustic and satellite transmitters on sea turtles in future tracking studies where continuous monitoring with fine-scale resolution at prescribed spatial locations (monitored by acoustic arrays) is desirable.
Year-round residence near Canaveral and being “first in line” for vernal breeding offers a distinct reproductive advantage given polyandry documented for this species (Moore and Ball 2002). Shorter migration distances for resident males should conserve energy that could instead be utilized for building mass and (seasonally) reproductive output; thus, enabling them to mate sooner during the season as suggested by all inactive males being resident and a more languid dispersal of residents relative to migrants in our study. At Port Canaveral and in Laganas Bay, Greece, peak male aggregation has been observed in April (Henwood 1987; Schofield et al. 2006; Hays et al. 2010); however, at both study locations male aggregation begins as early as February (Henwood 1987; Schofield et al. 2009; Hays et al. 2010) with mating commencing shortly thereafter (Wibbels et al. 1990; Schofield et al. 2006; Hays et al. 2010). By curbing energy needed for reproductive migrations, residents may also be more likely to breed annually which has been reported for adult males in several species of sea turtle (Balazs 1983; Wibbels et al. 1990; James et al. 2005), but that we observed much less commonly (Blanvillain et al. 2008).
Resident male were smaller than migrant males and exhibited a haplotype distribution consistent with haplotype distributions reported for south Florida nesting beaches (Encalada et al. 1998; Bowen et al. 2004). As such, smaller sizes associated with residents may reflect genetically smaller turtles of similar age as opposed to turtles with similar growth rates but from younger cohorts. Therefore, as technological capabilities enable minimally invasive age assessments, characterization of the age structure of loggerheads at this important mating aggregation should be considered a high priority, particularly given that adult male loggerheads > 95 cm in the current study were almost exclusively migrants and were collected less frequently than at this location during 1978–1984 (Henwood 1987) and 1993 (Dickerson et al. 1995). Alternatively, smaller size of residents may reflect differential growth as a function of foraging habitat (Bjorndal et al. 2000), particularly given consistent movements to the middle continental shelf after the breeding period where loggerheads have been occasionally sighted (in aggregate) inshore of the western wall of the Gulf Stream during aerial surveys (Hoffman and Fritts 1982; Schroeder and Thompson 1987). Furthermore, given consistent movement of resident males to the middle and outer continental shelf after the breeding period and only modest amounts of time spent at the water surface, we suspect that the abundance of resident adult male loggerheads in this region be under reported in offshore aerial surveys particularly given evasive movements reported for loggerheads in response to low-flying aircraft (Frick et al. 2000).
Migrant and resident adult male loggerheads dispersed concurrently; however, neither demographic indicators nor testosterone were able to account for any of the variance among actual dispersal dates which spanned 1 month for migrants and 2 months for residents. Rapid and concurrent dispersal of migrant and resident adult male loggerheads during May is also reported for a smaller sample size in Laganas Bay, Greece in May (Schofield et al. 2010a, b), where mating is also temporally constricted (Schofield et al. 2006). Wibbels et al. (1987) previously suggested that decreasing testosterone levels during the month of May as the likely stimulus for initiating dispersal given similar decline in testosterone levels within individuals resampled during mating in seminatural conditions for both Kemps ridley (Rostal et al. 1998) and green (Licht et al. 1980) sea turtles. Blanvillain et al. (2008) determined that while high testosterone levels certainly indicate reproductive activity, low testosterone levels do not necessarily indicate reproductive inactivity. We concur with Wibbels et al. (1987) that a hormonal change is a reasonable explanation for rapid dispersal; however, given variability in testosterone levels among individuals, lack of a definitive relationship between testosterone, and the number of days elapsed prior to commencing dispersal, we further suggest that physiological thresholds for signaling dispersal must also vary among individuals as well.
Visual observations of mounted pairs in turbid coastal waters that characterize the Southeast United States have led to the supposition that mating occurs at the surface; however, with few exceptions our data did not suggest frequent utilization of surface waters. In the current study, transmitter measurements of surfacing behavior were similar between reproductively active and inactive males during the breeding period, and data suggested that loggerheads rarely spent more than 10% of their time at the surface, also corroborated by relatively stable temperature readings. Median dive duration for adult male loggerheads was approximately 30 min, also inconsistent with the suggestion of extended time spent at the surface. Reproductively active males made shorter dives than inactive males which may reflect the energetic demands of breeding males (Jessop et al. 2004), particularly while in pursuit of females who are being approached by multiple competing males (Booth and Peters 1972; Schofield et al. 2006). Schofield et al. (2006) reported that mounted loggerhead pairs on the seafloor in Laganas Bay, Greece more commonly occurred under higher sea states and lower visibility; however, our data indicated longer dive durations at higher wind speeds both during and after the breeding period. As such, increased sightings of loggerhead sea turtles on the seafloor at higher wind speeds reported by Schofield et al. (2006) may have simply reflected a behavioral response to sea state rather than a reproductive strategy. Nevertheless, given dive and submergence patterns in the present study and that sea turtle copulation may last up to six (Booth and Peters 1972) to ten (Miller 1996) hours, we suggest that much breeding activity occurs underwater and that mounted pairs are observed at the surface primarily when at least one turtle returns to the surface to breathe or to end copulation (Booth and Peters 1972; Schofield et al. 2006).
Resident males experienced water temperatures 18–28°C, a moderate range for ectotherms. Dive duration increased through autumn as photoperiod and water temperature declined, consistent with observations for this species elsewhere (Hochscheid et al. 2007, 2010). As such, reduced data collection for formerly active males during autumn may also explain why formerly active males appeared to make shorter dives than inactive males after the breeding period. Resident males were not monitored during winter, but likely remained resident given that temperatures recorded at the Canaveral Offshore Buoy during winter were comparable to known overwintering temperatures for this species (Coles and Musick 2000; Hochscheid et al. 2007; Mansfield et al. 2009; Schofield et al. 2009). Hochscheid et al. (2007) also report that activity is more common than dormancy for juvenile loggerheads in the Mediterranean Sea during winter; however, the Port Canaveral shipping channel is one of the few if not the only location in the world where loggerhead brumation is reported (Carr et al. 1980), offering yet another thermal advantage to resident adult male loggerheads during exceptionally cold winters.
The current study represents the first satellite telemetry study of distribution and behavior of adult male loggerhead sea turtles at this important breeding aggregation located 40 km north of the second largest loggerhead rookery in the world (NMFS and USFWS 2008). The data collected during this study constitute only the third attempt during the past three decades to collect distributional data for adult male loggerheads at this location, and only the first at this location to monitor the movements of adult male sea turtles of known reproductive activity level (Blanvillain et al. 2008). The data set reported herein also represents the single greatest number of adult male sea turtles of any species cumulatively outfitted with telemetry devices at a breeding location anywhere in the world, with prior studies involving one (Sakamoto et al. 1997; Hatase et al. 2002) but upwards to 11 individuals (Plotkin et al. 1996; James et al. 2005; van Dam et al. 2008; Schofield et al. 2010a, b). The historical lack of distributional information for adult male loggerheads is attributed to logistical and fiscal constraints associated with capturing males at sea and outfitting them with telemetry devices; however, given recent successes with long-term monitoring of adult male sea turtles collected from breeding (Plotkin et al. 1996; Shaver et al. 2005; van Dam et al. 2008; Schofield et al. 2009, 2010a, b; Hays et al. 2010; this study) and foraging (James et al. 2005; Sherrill-Mix and James 2008) aggregations, we anticipate greater data collection for adult male sea turtles in the future.
As the capacity to identify and monitor breeding locations increases, and the technology to track and manage data for tagged turtles improves, we anticipate that the data set reported here will provide a valuable reference for a proliferation of similar research studies at key breeding sites around the world. Long-term data on the relative abundance and demographic distributions of loggerheads (predominantly juveniles) exist for numerous locations on multiple foraging grounds, several of which (Florida Bay in the USA, Heron Atoll in Australia, Laganas Bay in Greece) also seasonally support breeding adult male loggerheads. As such, we hope that in time the amount of data collected available for adult male sea turtles will eventually rival historically better studied adult females and juveniles (Godley et al. 2008). Many similarities were noted for adult male loggerheads in this study and Laganas Bay with respect to seasonal onset and cessation of activity; however, notable differences included a greater proportion and wider spatial distribution of resident individuals. Given these differences, we recommend that future research at our study location collect data on operational sex ratios which have been documented for Laganas Bay (Hays et al. 2010) and assessment of inter- and intra-breeding periodicity and reproductive lifespan (NMFS and USFWS 2008) which have not been documented at either location.
References
Balazs GH (1983) Recovery records of adult green turtles observed originally tagged at French Frigate Shoals, Northwestern Hawaiian Islands. NOAA-TM-NMFS-SWFC-36, 47p
Bjorndal KA, Bolten AB, Chaloupka MY (2000) Green turtle somatic growth model: evidence for density dependence. Ecol Appl 10:269–282
Blanvillain G, Pease AP, Segars AL, Rostal DC, Richards AJ, Owens DW (2008) Comparing methods for the assessment of reproductive activity in adult male loggerhead sea turtles Caretta caretta at Cape Canaveral, Florida. Endanger Species Res 6:75–85
Booth J, Peters JA (1972) Behavioural studies on the green turtle (Chelonia mydas) in the sea. Anim Behav 20(4):808–812
Bowen BW, Bass AL, Chow SM, Bostrom M et al (2004) Natal homing in juvenile loggerhead turtles (Caretta caretta). Mol Ecol 13:3797–3808
Carr A, Ogren L, McVea C (1980) Apparent hibernation by the Atlantic loggerhead turtle Caretta caretta off Cape Canaveral, Florida. Biol Conserv 19:7–14
Coles WC, Musick JA (2000) Satellite sea surface temperature analysis and correlation with sea turtle distribution off North Carolina. Copeia 2:551–554
Coyne MS, Godley BJ (2005) Satellite tracking and analysis tool: an integrated system for archiving, analyzing and mapping animal tracking data. Mar Ecol Prog Ser 301:1–7
Coyne M, Landry AM Jr (2007) Population sex ratios and its impact on population models. In: Plotkin PT (ed) Biology and conservation of ridley sea turtles. Johns Hopkins University Press, Baltimore, pp 191–211
Dickerson DD, Reine KJ, Nelson DA, Dickerson CE Jr (1995) Assessment of sea turtle abundance in six South Atlantic US channels. Miscellaneous Paper EL-95-5, US Army Engineer Waterways Experiment Station, Vicksburg
Encalada SE, Bjorndal KA, Bolten AB, Zurita JC, Schroeder B, Possardt E, Sears CJ, Bowen BW (1998) Population structure of loggerhead turtle (Caretta caretta) nesting colonies in the Atlantic and Mediterranean as inferred from mitochondrial DNA control region sequences. Mar Biol 130:567–575
Frick MG, Slay CK, Quinn CA, Windham-Reid A, Duley PA, Ryder CM, Morse LJ (2000) Aerial observations of courtship behaviour in loggerhead sea turtles (Caretta caretta) from South Eastern Georgia and Northeastern Florida. J Herpetol 34(1):153–158
Godley BJ, Broderick AC, Frauenstein R, Glen F, Hays GC (2002) Reproductive seasonality and sexual dimorphism in green turtles. Mar Ecol Prog Ser 226:125–133
Godley BJ, Blumenthal JM, Broderick AC, Coyne MS, Godfrey MH, Hawkes LA, Witt MJ (2008) Satellite tracking of sea turtles: where have we been and where do we go next? Endanger Spec Res 4(1–2):3–22
Hatase H, Matsuzawa Y, Sakamoto W, Baba N, Miyawaki I (2002) Pelagic habitat use of an adult Japanese male loggerhead turtle Caretta caretta examined by the Argos satellite system. Fish Sci 68:945–947
Hawkes LA, Broderick AC, Godfrey MH, Godley BJ (2007) Investigating the potential impacts of climate change on a marine turtle population. Glob Change Biol 13(5):923–932
Hays GC, Fossette S, Katselidis KA, Schofield G, Gravenor MB (2010) Breeding periodicity for male sea turtles, operational sex ratios, and implications in the face of climate change. Conserv Biol 24(6):1636–1643
Henwood TA (1987) Movements and seasonal changes in loggerhead turtle Caretta caretta aggregations in the vicinity of Cape Canaveral, Florida (1978–1984). Biol Conserv 40:191–202
Hochscheid S, Bentivegna F, Bradai MN, Hays GC (2007) Overwintering behavior in sea turtles: dormancy is optional. Mar Ecol Prog Ser 340:287–298
Hochsheid S, Bentivegna F, Hamza A, Hays GC (2010) When surfacers do not dive: multiple significance of extended surface times in marine turtles. J Exp Biol 213:1328–1337
Hoffman W, Fritts TH (1982) Sea turtle distribution along the boundary of the Gulf stream current off eastern Florida. Herpetologica 38(3):405–409
James MC, Eckert SA, Myers RA (2005) Migratory and reproductive movements of male leatherback turtles (Dermochelys coriacea). Mar Biol 147:845–853
Jessop TS, Hamann M, Limpus CJ (2004) Body condition and physiological changes in male green sea turtles during breeding. Mar Ecol Prog Ser 276:281–288
Kemmerer AJ, Timko RE, Burkett SB (1983) Movement and surfacing patterns of loggerhead sea turtles in and near Canaveral Channel, Florida (September and October 1981). NOAA Tech Mem NMFS-SEFSC-112
Lazell JD (1980) New England waters: critical habitat for marine turtles. Copeia 1980:290–295
Licht P, Rainey W, Cliffton K (1980) Serum gonadotropin and steroids associated with breeding activities in the green sea turtle, Chelonia mydas: II. Mating and nesting in natural populations. Gen Comp Endocrinol 40(1):116–122
Luschi P, Hays GC, Del Seppia C, Marsh R, Papi F (1998) The navigational feats of green sea turtles migrating from Ascension Island investigated by satellite telemetry. Proc R Soc B Biol Sci 265:2279–2284
Mansfield KL, Saba VS, Keinath JA, Musick JA (2009) Satellite telemetry reveals dichotomy in migration strategies among juvenile loggerhead turtles in the Northwest Atlantic. Mar Biol 156:2555–2570
Margaritoulis D (2005) Nesting activity and reproductive output of loggerhead sea turtles, Caretta caretta, over 19 seasons (1984–2002) at Laganas Bay, Zakynthos, Greece: the largest rookery in the Mediterranean. Chel Cons Biol 4(4):916–929
Miller JD (1996) Reproduction in sea turtles. In: Lutz PL, Musick JA (eds) The biology of sea turtles. CRC Press, Boca Raton, pp 51–82
Moore MK, Ball RM (2002) Multiple paternity in loggerhead turtle (Caretta caretta) nests on Melbourne Beach, Florida: a microsatellite analysis. Mol Ecol 11:281–288
National Marine Fisheries Service (NMFS) and US Fish and Wildlife Service (USFWS) (2008) Recovery plan for the Northwest Atlantic Population of the Loggerhead Sea Turtle (Caretta caretta), 2nd revision, Silver Spring. Accessed 20 May 2011. http://www.nmfs.noaa.gov/pr/pdfs/recovery/turtle_loggerhead_atlantic.pdf
Owens DW (1980) The comparative reproductive physiology of sea turtles. Am Zool 20:549–563
Owens DW, Ruiz GJ (1980) New methods of obtaining blood and cerebrospinal fluid from marine turtles. Herpetologica 36:17–20
Plotkin PT, Owens DW, Byles R, Patterson RM (1996) Departure of male olive ridley turtles (Lepidochelys olivacea) from a nearshore breeding ground. Herpetologica 52:1–7
Polovina JJ, Kobayashi DR, Parker DM, Seki MP, Balazs GH (2000) Turtles on the edge: movement of loggerhead turtles (Caretta caretta) along oceanic fronts, spanning longline fishing grounds in the central North Pacific, 1997–1998. Fish Oceanogr 9:71–82
Roberts MA, Anderson CJ, Stender B, Segars A, Whitaker JD, Grady JM, Quattro JM (2005) Estimated contribution of Atlantic Coastal loggerhead turtle nesting populations to offshore feeding aggregations. Conserv Genet 6:133–139
Rostal DC, Owens DM, Grumbles JS, MacKenzie DS, Amoss MS Jr (1998) Seasonal reproductive cycle of the Kemp’s ridley sea turtle (Lepidochelys kempi). Gen Comp Endocrinol 109(2):232–243
Sakamoto W, Bando T, Arai N, Baba N (1997) Migration path of the adult female and male loggerhead turtles Caretta caretta determined through satellite telemetry. Fish Sci 63(4):547–552
Schmid JR (1995) Marine turtle populations on the east-central coast of Florida: results of tagging studies at Cape Canaveral, Florida, 1986–1991. Fish Bull 93:139–151
Schofield G, Katselidis KA, Dimopoulos P, Pantis JD, Hays GC (2006) Behaviour analysis of the loggerhead sea turtle (Caretta caretta) from direct in-water observation. Endanger Species Res 2:71–79
Schofield G, Lilley MKS, Bishop CM, Brown P, Katselidis KA, Dimopoulos P, Pantis JD, Hays GC (2009) Conservation hotspots: implications of intense spatial area use by breeding male and female loggerheads at the Mediterranean’s largest rookery. Endanger Species Res 10:191–202
Schofield G, Hobson VJ, Fossette S, Lilley MKS, Katselidis K, Hays GC (2010a) Fidelity to foraging sites, consistency of migration routes and habitat modulation of home range by sea turtles. Divers Distrib 16(5):840–853
Schofield G, Hobson VJ, Lilley MKS, Katselidis K, Bishop CM, Brown P, Hays GC (2010b) Inter-annual variability in the home range of breeding turtles: implications for current and future conservation management. Biol Conserv 143:722–730
Schroeder BA, Thompson NB (1987) Distribution of the loggerhead turtle, Caretta caretta, and the leatherback turtle, Dermochelys coriacea, in the Cape Canaveral Florida Area: Results of Aerial Surveys. In: Witzell WN (ed) Ecology of east Florida sea turtles. Proceedings of the Cape Canaveral, Florida Sea turtle workshop, pp 45–53. NOAA Technical Report NMFS 53
Shaver DJ, Schroeder BA, Byles RA, Burchfield PM, Pena J, Marquez R, Martinez HJ (2005) Movements and home ranges of adult male Kemp’s ridley sea turtles (Lepidochelys kempii) in the Gulf of Mexico investigated by satellite telemetry. Chelonian Conserv Biol 4:817–827
Sherrill-Mix SA, James MC (2008) Evaluating potential tagging effects on leatherback sea turtles. Endanger Species Res 4:187–193
Standora EA, Spotilla JR (1985) Temperature dependent sex determination in sea turtles. Copeia 1985:711–722
Standora EA, Eberle MD, Edbauer JM, Ryder TS, Williams KL, Morreale SJ, Bolten AB (1993) Diving behavior, daily movements, and homing of loggerhead turtles (Caretta caretta) at Cape Canaveral, Florida, March and April 1993. Final contract report to US Army Engineer Waterways Experiment Station, Buffalo State College, Buffalo; Okeanos Ocean Research Foundation, Inc., Hampton Bays, and University of Florida, Gainesville
van Dam RP, Diez CE, Balazs GH, Colón LAC, McMillan WO, Schroeder B (2008) Sex- specific migration patterns of hawksbill turtles from Mona Island, Puerto Rico. Endanger Spec Res 4(1–2):85–94
Wibbels T, Owens DW, Amoss MS (1987) Seasonal changes in the serum titers of loggerhead sea turtles captured along the Atlantic Coast of the United States. In: Witzel W (ed) Ecology of East Florida Sea Turtles, pp 59–64. NOAA Tech Report NMFS-53
Wibbels T, Owens DW, Limpus CJ, Reed PC, Amoss MS (1990) Seasonal changes in serum gonadal steroids associated with migration, mating and nesting in loggerhead sea turtle (Caretta caretta). Gen Comp Endocrinol 79:154–164
Witherington BE, Ehrhart LM (1989) Hypothermic stunning and mortality of marine turtles in the Indian River Lagoon System, Florida. Copeia 1989(3):696–703
Acknowledgments
Crucial field support was provided by Milliken Seafood, Cape Canaveral Seafood, C. Storts (Atlantic Animal Clinic) and L. Liguori, M. Higgins and J. Dickey at the University of Georgia Marine Extension Service. We thank the following for assistance in their “backyard”: B. Schroeder and S. Epperly (National Marine Fisheries Service, NMFS); A. Meylan, A. Foley, B. Witherington, T. Hirama and C. Eaton (Florida Fish and Wildlife Conservation Commission, FWC); L. Ehrhart and D. Bagley (University of Central Florida, UCF). K. Mazzarella (Mote Marine Laboratory), D. Griffin (South Carolina Department of Natural Resources, SCDNR), J. Keller (National Institute of Standards and Technology), K. Thorvalson (South Carolina Aquarium), and A.M. Lee and D. Stanford contributed long hours in the field. J. Schwenter (SCDNR), S. Hopkins-Murphy (SCDNR, retired), K. Mansfield (NMFS), B. Godley (University of Exeter), S. Ceriani (UCF), and anonymous reviewers provided critical edits to this manuscript. Funding was provided by NOAA grant NA03NMF4720281 and managed by J. Brown (NMFS) and E. Heyward (SCDNR). Research was authorized by Section 10(a)(1)(A) permit #1540 and Florida Marine Turtle Permit #163, and we thank P. Opay and K. Swails (NMFS) and R. Trindell and M. Koperski (FWC) for their support. This manuscript is contribution #685 of the Marine Resources Division of the South Carolina Department of Natural Resources and #375 of the College of Charleston, Grice Marine Biology Laboratory.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. D. R. Houghton.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Arendt, M.D., Segars, A.L., Byrd, J.I. et al. Distributional patterns of adult male loggerhead sea turtles (Caretta caretta) in the vicinity of Cape Canaveral, Florida, USA during and after a major annual breeding aggregation. Mar Biol 159, 101–112 (2012). https://doi.org/10.1007/s00227-011-1793-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-011-1793-5