Abstract
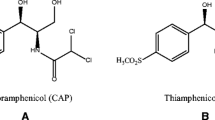
Different highly selective sorbents have been evaluated for the treatment of food and biological samples to determine chloramphenicol residues by ion mobility spectrometry (IMS). Combination of a selective solid-phase extraction (SPE) and dispersive liquid-liquid microextraction allowed a highly sensitive determination of chloramphenicol in water, milk, honey, and urine samples. The performance of selective SPE supports such as immunoaffinity chromatography (IAC) and molecular imprinted polymers (MIP) have been compared in terms of selectivity, sensitivity, trueness, precision, and reusability. Quantitative recoveries were obtained for chloramphenicol residues, ranging from 91 to 123 % for water, from 99 to 120 % for skimmed milk, and from 95 to 124 % for urine using IAC-IMS and MIP-IMS methods. Quantitative recoveries (from 88 to 104 %) were also achieved for honey samples using IAC-IMS, but low recoveries were obtained using MIP-IMS. The limit of quantification was set at 0.1 μg L−1 which is lower than the minimum required performance limit established by the EU. The proposed methodology is a simple and cost affordable alternative to chromatography methods for the highly sensitive and selective analysis of chloramphenicol residues in food and urine.

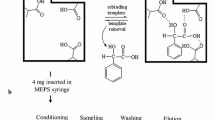
Scheme for chloramphenicol determination by selective solid-phase extraction and ion mobility spectrometry



Similar content being viewed by others
References
Eiceman GA. Ion-mobility spectrometry as a fast monitor of chemical composition. TrAC Trend Anal Chem. 2002;21:259–75.
Armenta S, Alcala M, Blanco M. A review of recent, unconventional applications of ion mobility spectrometry (IMS). Anal Chim Acta. 2011;703:114–23.
Harrington PD, Reese ES, Rauch PJ, Hu LJ, Davis DM. Interactive self-modeling mixture analysis of ion mobility spectra. Appl Spectrosc. 1997;51:808–16.
Asbury GR, Hill HH. Evaluation of ultrahigh resolution ion mobility spectrometry as an analytical separation device in chromatographic terms. J Microcolumn. 2000;12:172–8.
Zamora D, Blanco M. Improving the efficiency of ion mobility spectrometry analyses by using multivariate calibration. Anal Chim Acta. 2012;726:50–6.
Armenta S, Blanco M. Pros and cons of benzodiazepines screening in human saliva by ion mobility spectrometry. Anal Bioanal Chem. 2011;401:1935–48.
Soleimani M, Azam M, Azimi M, Borhani K. SPE-IMS as a new analysis technique for identification and quantification of metalaxyl residue in cucumber. Ital J Food Sci. 2012;24:3–8.
Holopainen S, Luukkonen V, Nousiainen M, Sillanpää M. Determination of chlorophenols in water by headspace solid phase microextraction ion mobility spectrometry (HS-SPME-IMS). Talanta. 2013;114:176–82.
Kalhor H, Ameli A, Alizadeh N. Electrochemically controlled solid-phase micro-extraction of proline using a nanostructured film of polypyrrole, and its determination by ion mobility spectrometry. Microchim Acta. 2013;180:783–9.
Lokhnauth JK, Snow NH. Stir-bar sorptive extraction and thermal desorption-ion mobility spectrometry for the determination of trinitrotoluene and l,3,5-trinitro-l,3,5-triazine in water samples. J Chromatogr A. 2006;1105:33–8.
Armenta S, Garrigues S, de la Guardia M, Brassier J, Alcalà M, Blanco M. Analysis of ecstasy in oral fluid by ion mobility spectrometry and infrared spectroscopy after liquid–liquid extraction. J Chromatogr A. 2015;1384:1–8.
Márquez-Sillero I, Aguilera-Herrador E, Cárdenas S, Valcárcel M. Determination of 2,4,6-tricholoroanisole in water and wine samples by ionic liquid-based single-drop microextraction and ion mobility spectrometry. Anal Chim Acta. 2011;702:199–204.
Saraji M, Jafari MT, Sherafatmand H. Hollow fiber-based liquid–liquid–liquid microextraction combined with electrospray ionization-ion mobility spectrometry for the determination of pentazocine in biological samples. J Chromatogr A. 2010;1217:5173–8.
Holopainen S, Nousiainen M, Sillanpää MET, Anttalainen O. Sample-extraction methods for ion-mobility spectrometry in water analysis. TrAC Trend Anal Chem. 2012;37:124–34.
Khalesi M, Sheikh-Zeinoddin M, Tabrizchi M. Determination of ochratoxin A in licorice root using inverse ion mobility spectrometry. Talanta. 2011;83:988–93.
Sheibani A, Tabrizchi M, Ghaziaskar HS. Determination of aflatoxins B1 and B2 using ion mobility spectrometry. Talanta. 2008;75:233–8.
Armenta S, de la Guardia M, Abad-Fuentes A, Abad-Somovilla A, Esteve-Turrillas FA. Off-line coupling of multidimensional immunoaffinity chromatography and ion mobility spectrometry: a promising partnership. J Chromatogr A. 2015;1426:110–7.
Jafari MT, Rezaei B, Zaker B. Ion mobility spectrometry as a detector for molecular imprinted polymer separation and metronidazole determination in pharmaceutical and human serum samples. Anal Chem. 2009;81:3585–91.
Rezaei B, Jafari MT, Khademi R. Selective separation and determination of primidone in pharmaceutical and human serum samples using molecular imprinted polymer-electrospray ionization ion mobility spectrometry (MIP-ESI-IMS). Talanta. 2009;79:669–75.
Jafari MT, Badihi Z, Jazan E. A new approach to determine salicylic acid in human urine and blood plasma based on negative electrospray ion mobility spectrometry after selective separation using a molecular imprinted polymer. Talanta. 2012;99:520–6.
Jafari MT, Kamfirozi M, Jazan E, Ghoreishi SM. Selective extraction and analysis of pioglitazone in cow plasma using a molecularly imprinted polymer combined with ESI ion mobility spectrometry. J Sep Sci. 2014;37:573–9.
Lu W, Li H, Meng Z, Liang X, Xue M, Wang Q, et al. Detection of nitrobenzene compounds in surface water by ion mobility spectrometry coupled with molecularly imprinted polymers. J Hazard Mat. 2014;280:588–94.
European Food Safety Authority. Scientific opinion on chloramphenicol in food and feed. EFSA J. 2014;12–3907:1–145.
Food and Agriculture Organization of the United Nations/World Health Organization. Summary report of the sixty-second meeting of JECFA. FAO Food Nutrit Papers. 2004;41–6:1–12.
Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–7.
Esteve-Turrillas FA, Mercader JV, Agulló C, Abad-Somovilla A, Abad-Fuentes A. Development of immunoaffinity columns for pyraclostrobin extraction from fruit juices and analysis by liquid chromatography with UV detection. J Chromatogr A. 2011;1218:4902–9.
West C, Baron G, Minet J. Detection of gunpowder stabilizers with ion mobility spectrometry. J Forensic Sci Int. 2007;166:91–101.
Jafari MT, Khayamian T, Shaer V, Zarei N. Determination of veterinary drug residues in chicken meat using corona discharge ion mobility spectrometry. Anal Chim Acta. 2007;581:147–53.
Picó Y. Food contaminants and residue analysis. Elsevier ISBN: 978-0-444-53019-6; 2008.
Esteve-Turrillas FA, Abad-Somovilla A, Quiñones-Reyes G, Agulló C, Mercader JV, Abad-Fuentes A. Monoclonal antibody-based immunoassays for cyprodinil residue analysis in QuEChERS-based fruit extracts. Food Chem. 2015;187:530–6.
Acknowledgments
Authors gratefully acknowledge the financial support of the Ministerio de Economía y Competitividad (AGL2012-39965-C02-01-02/ALI, CTQ-2012-38635, and CTQ-2014-52841) and Generalitat Valenciana (PROMETEO-II 2014-077).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study has been approved by the appropriate ethics committee and has been performed in accordance with the ethical standards.
Conflict of interest
The authors declare no competing financial or nonfinancial interest.
Additional information
These results were presented as a poster communication at the “XXV Reunión Nacional de Espectroscopía—VIII Congreso Ibérico de Espectroscopía” (XXV RNE-VIII CIE) held in Alicante (Spain) in July 2016 and it won the Poster Prize for excellent presentation of particularly significant innovative analytical research.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 346 kb)
Rights and permissions
About this article
Cite this article
Armenta, S., de la Guardia, M., Abad-Fuentes, A. et al. Highly selective solid-phase extraction sorbents for chloramphenicol determination in food and urine by ion mobility spectrometry. Anal Bioanal Chem 408, 8559–8567 (2016). https://doi.org/10.1007/s00216-016-9995-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-016-9995-9