Abstract
Celiac disease (CD) is a permanent gastrointestinal disorder characterized by the intolerance to a group of proteins called gluten present in wheat, rye, barley, and possibly oats. The only therapy is a strict lifelong gluten-free diet. The standard method for gluten determination in foods produced for CD patients is the R5-enzyme-linked immunosorbent assay (ELISA) as proposed by the recent Codex Alimentarius Draft Revised Standard. This test is based on the determination of prolamins, the alcohol-soluble proteins of gluten, and is available as a sandwich ELISA for intact proteins and as a competitive ELISA for gluten-derived peptides. While the suitability of the sandwich ELISA including a wheat prolamin (gliadin) reference for calibration has been shown by various studies and a ring test, the competitive ELISA still lacks a convenient reference for the quantitation of gluten peptides in fermented cereal foods (e.g., sourdough products, starch syrup, malt extracts, beer). Therefore, the aim of the present study was to prepare a suitable reference for the quantitation of partially hydrolyzed gluten in fermented wheat, rye, and barley products. The prolamin fractions from barley (hordein) and rye (secalin) were isolated from corresponding flours by means of a modified preparative Osborne fractionation. The prolamin fraction from wheat was obtained as reference gliadin from the Prolamin Working Group. The prolamin fractions were successively digested by pepsin and trypsin or pepsin and chymotrypsin procedures, which have been used for CD-specific toxicity tests on cereal storage proteins for many years. The protein/peptide content (N × 5.7) of the prolamin fractions and digests, which was the basis for the calculation of the gluten content by means of ELISA, varied between 67.1% and 96.0%. The prolamin fractions and enzymatic digests were then tested for their response in both sandwich and competitive assays. Intact prolamins responded similarly in both ELISA showing no important differences between the cereals. In the case of digested proteins, however, the sandwich ELISA was considerably less sensitive than the competitive ELISA. The former provided approximately 40% and the latter 70% of the signal intensity obtained with the intact prolamins. Thus, the combination of the competitive ELISA and the enzymatic digests of prolamin fractions as reference was considered to be an adequate system for the analysis of partially hydrolyzed gluten. The limit of detection using a peptic-tryptic hordein digest as reference was 2.3 µg prolamin equivalent per milliliter, and the limit of quantitation was 6.7 µg prolamin equivalent per milliliter. This system was applied for the determination of gluten equivalents in five commercial beverages based on fermented cereals.

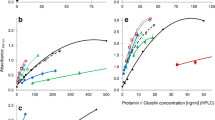
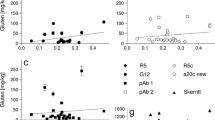
Schematic representation of a competitive ELISA (top) and examples for the measurement of the prolamin content of different samples and references (bottom)




Similar content being viewed by others
Notes
One-letter-code for amino acids
Abbreviations
- CD:
-
Celiac disease
- ELISA:
-
Enzyme-linked immunosorbent assay
- HMM:
-
High molecular mass
- HPLC:
-
High-performance liquid chromatography
- LOD:
-
Limit of detection
- LOQ:
-
Limit of quantitation
- M r :
-
Molecular mass (relative)
- PAGE:
-
Polyacrylamidegel electrophoresis
- PC:
-
Peptic-chymotryptic
- PT:
-
Peptic-tryptic
- PWG:
-
Prolamin working group
- RP:
-
Reversed-phase
- RT:
-
Room temperature
- SDS:
-
Sodium docecylsulfate
References
Rewers M (2005) Gastroenterol 128:47–51
Wieser H, Koehler P (2008) Cereal Chem 85:1–13
ALINORM 08/31/26, Appendix III (2008) Codex Alimentarius Commission, WHO, Rome
Valdes I, Garcia E, Llorente M, Mendez E (2003) Eur J Gastroen Hep 15:466–474
Van Eckert R, Berghofer E, Ciclitira PJ, Chirdo F, Denery-Papini S, Ellis HJ, Ferranti P, Goodwin P, Immer U, Mamone G, Mendez E, Mothes T, Novalin S, Osman A, Rumbo M, Stern M, Thorell L, Whim A, Wieser H (2006) J Cereal Sci 43:331–341
Immer U, Vela C, Mendez F, Janssen F (2003) In: Stern M (ed) Proceedings of the 17th meeting of the working group on prolamin analysis and toxicity. Verlag Wissenschaftliche Scripten, Zwickau, pp 23–33
Immer U, Haas-Lauterbach S (2004) In: Stern M (ed) Proceedings of the 18th meeting of the working group on prolamin analysis and toxicity. Verlag Wissenschaftliche Scripten, Zwickau, pp 23–36
R-Biopharm AG (2007) Ridoscreen® Gliadin competitive—Product Information
Wieser H, Antes S, Seilmeier W (1998) Cereal Chem 75:644–650
Frazer AC, Fletcher RF, Ross CAC, Shaw B, Sammons HG, Schneider R (1959) Lancet II:252–255
Kasarda DD, Woodard KM, Adalsteins AE (1998) Cereal Chem 75:70–71
Hädrich J, Vogelgesang J (1996) Deutsche Lebensmittel-Rundschau 92:341–350
Anonymus (2007) In: Stern M (ed) Proceedings of the 21th meeting of the working group on prolamin analysis and toxicity. Verlag Wissenschaftliche Scripten, Zwickau, p 160
Shewry PR, Tatham AS (1990) Biochem J 267:1–12
Gellrich C, Schieberle P, Wieser H (2003) Cereal Chem 80:102–109
Lange M, Vincze E, Wieser H, Schjoerring JK, Holm PB (2007) J Agric Food Chem 55:6074–6081
Burnouf T, Bietz JA (1985) Theor Appl Genet 70:610–619
Wieser H, Zimmermann G (2000) Eur Food Res Technol 210:324–330
Osman AA, Uhlig HH, Valdes I, Amin M, Mendez E, Mothes T (2001) Eur J Gastroen Hep 13:1189–1193
Kagnoff MF (2007) J Clin Invest 117:41–49
Acknowledgments
The authors thank Leibniz-Gemeinschaft (WGL) for financial support and Mrs. K. Schießer and Mrs. A. Axthelm for excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gessendorfer, B., Koehler, P. & Wieser, H. Preparation and characterization of enzymatically hydrolyzed prolamins from wheat, rye, and barley as references for the immunochemical quantitation of partially hydrolyzed gluten. Anal Bioanal Chem 395, 1721–1728 (2009). https://doi.org/10.1007/s00216-009-3080-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-009-3080-6