Abstract
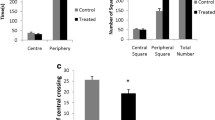
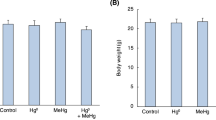
Previously we found that exposure to mercury vapor effectively induced metallothionein (MT) biosynthesis in rat brain. Although the induction of not only MT-I/II but also MT-III was evident, the induction rate of the latter was much lower than that of the former. The brain of an MT-null mouse lacks MT-I/II, but has MT-III. Here we examined the effects of sub-chronic pulse exposure to mercury vapor on the brain MT in MT-null mice and their wild type controls. MT-null and wild type mice were preliminarily exposed to mercury vapor for 2 weeks at 0.1 mg Hg/m3 for 1 h/day for 3 days a week, and then exposed for 11 weeks at 4.1 mg Hg/m3 for 30 min/day for 3 days a week. This exposure caused no toxic signs such as abnormal behavior or loss of body weight gain in the mice of either strain throughout the experimental period. Twenty-four hours after the termination of the exposure, mice were sacrificed and brain samples were subjected to mercury analysis, MT assay, and pathological examination. The MT-null mice showed lower accumulation of mercury in the brain than the wild type mice. Mercury exposure resulted in a 70% increase of brain MT in the wild type mice, which was mostly accounted for by the increase in MT-I/II. On the other hand, the brain MT in the MT-null mice increased by 19%, suggesting less reactivity of the MT-III gene to mercury vapor. Although histochemical examination revealed silver-mercury grains in the cytoplasm of nerve cells and glial cells throughout the brains of both strains, no significant difference was observed between the two strains.






Similar content being viewed by others
References
Cai L, Hammond RR, Cherian MG (1999) Metallothionein expression and radiation protection in primary human CNS cultures. Toxicol Sci 48[Suppl 1]:290–291
Chung RS, Vickers JC, Chuah MI, Eckhardt BL, West AK (2002) Metallothionein-III inhibits initial neurite formation in developing neurons as well as postinjury, regenerative neurite sprouting. Exp Neurol 178:1–12
Danscher G, Møller-Madsen B (1985) Silver amplification of mercury sulfide and selenide: a histochemical method for light and electron microscopic localization of mercury in tissue. J Histochem Cytochem 33:219–228
Ebadi M (1986) Characterization of a metallothionein-like protein in rat brain. Biol Trace Elem Res 11:101–116
Erickson JC, Sewell AK, Jensen LT, Winge DR, Palmiter RD (1994) Enhanced neurotrophic activity in Alzheimer’s disease cortex is not associated with down-regulation of metallothionein-III (GIF). Brain Res 646:297–304
Hirayama K (1985) Effects of combined administration of thiol compounds and methylmercury chloride on mercury distribution in rats. Biochem Pharmacol 34:2030–2032
Hozumi I, Inuzuka T, Tsuji S (1998) Brain injury and growth inhibitory factor (GIF)—a minireview. Neurochem Res 23:319–328
Irie Y, Keung WM (2001) Metallothionein-III antagonizes the neurotoxic and neurotrophic effects of amyloid beta peptides. Biochim Biophys Res Commun 282:416–420
Irie Y, Keung WM (2003) Anti-amyloid beta activity of metallothionein-III is different from its neuronal growth inhibitory activity: structure-activity studies. Brain Res 960:228–234
Itoh M, Ebadi M, Swanson S (1983) The presence of zinc binding proteins in brain. J Neurochem 41:823–829
Kobayashi H, Uchida Y, Ihara Y, Nakajima K, Kohsaka S, Miyatake T and Tsuji S (1993) Molecular cloning of rat growth inhibitory factor cDNA and the expression in the central nervous system. Mol Brain Res 19:188–194
Kramer KK, Liu J, Choudhuri S, Klaassen CD (1996) Induction of metallothionein mRNA and protein in murine astrocyte cultures. Toxicol Appl Pharmacol 136:94–100
Lee JY, Kim JH, Palmiter RD, Koh JY (2003) Zinc released from metallothionein-iii may contribute to hippocampal CA1 and thalamic neuronal death following acute brain injury. Exp Neurol 184:337–347
Miyazaki I, Asanuma M, Higashi Y, Sogawa CA, Tanaka K, Ogawa N (2002) Age-related changes in expression of metallothionein-III n rat brain. Neurosci Res 43:323–333
Montoliu C, Monfort P, Carrasco J, Palacios O, Capdevila M, Hidalgo J, Felipo V (2000) Metallothionein-III prevents glutamate and nitric oxide neurotoxicity in primary cultures of cerebellar neurons. J Neurochem 75:266–273
Naganuma A, Satoh M, Imura N (1987) Prevention of lethal and renal toxicity of cis-diamminedichloroplatinum (II) by induction of metallothionein synthesis without compromising its antitumor activity in mice. Cancer Res 47:983–987
Nishimura N, Nishimura H, Ghaffar A, Tohyama C (1992) Localization of metallothionein in the brain of rat and mouse. J Histochem Cytochem 40:309–315
Ono S, Cai L, Cherian MG (1998) Effects of gamma radiation on levels of brain metallothionein and lipid peroxidation in transgenic mice. Radiation Res 150:52–57
Ono S, Cai L, Koropatnick J, Cherian MG (2000) Radiation exposure does not alter metallothionein III isoform expression in mouse brain. Biol Trace Elem Res 74:23–30
Pamphlett R, Waley P (1996) Uptake of inorganic mercury by the human brain. Acta Neuropathol 92:525–527
Palmiter RD, Findley SD, Whitmore TE and Durnam DM (1992) MT-III, a brain-specific member of the metallothionein gene family. Proc Natl Acad Sci U S A 89:6333–6337
Rising L, Vitarella D, Kimerberg HK and Aschner M (1995) Metallothionein induction in neonatal rat primary astrocyte cultures protects against methylmercury cytotoxicity. J Neurochem 65:1562–1568
Uchida Y, Takio K, Titani K, Ihara Y and Tomonaga M (1991) The growth inhibitory factor that is deficient in the Alzheimer’s disease brain is a 68 amino acid metallothionein-like protein. Neuron 7:337–347
Yasutake A, Nakano A, Hirayama K (1998) Induction by mercury compounds of brain metallothionein in rats: Hg0 exposure induces long-lived brain metallothionein. Arch Toxicol 72:187–191
Yasutake A, Satoh M, Tohyama C, Hirayama K (1999) Selective and simple quantification of metallothionein III in mouse brain. J Health Sci 45:222–225
Yasutake A, Nagano M, Hirayama K (2003) Alterations of metallothionein isomers in Hg0-exposed rat brain. Arch Toxicol 77:12–16
Yoshida M, Satoh M, Shimada A, Yasutake A, Sumi Y, Tohyama C (1999a) Pulmonary toxicity caused by acute exposure to mercury vapor is enhanced in metallothionein-null mice. Life Sci 64:1861–1867
Yoshida M, Satoh M, Yasutake A, Shimada A, Sumi Y, Tohyama C (1999b) Distribution and retention of mercury in metallothionein-null mice after exposure to mercury vapor. Toxicology 139:129–136
You HJ, Oh DH, Choi CY, Lee DG, Hahm KS, Moon AR, Jeong HG (2002) Protective effect of metallothionein-III on DNA damage in response to reactive oxygen species. Biochim Biophys Acta 1573:33–38
Zheng H, Berman NE, Klaassen CD (1995) Chemical modulation of metallothionein I and III mRNA in mouse brain. Neurochem Int 27:43–58
Acknowledgements
The authors wish to thank Ms. M. Ogata and Ms. A. Hyodo for their technical assistance in the Hg analysis. The experimental protocol was approved by the Ethics Committee for Research on Animals in the National Institute for Minamata Disease.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yasutake, A., Sawada, M., Shimada, A. et al. Mercury accumulation and its distribution to metallothionein in mouse brain after sub-chronic pulse exposure to mercury vapor. Arch Toxicol 78, 489–495 (2004). https://doi.org/10.1007/s00204-004-0572-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-004-0572-1