Abstract
Summary
The calcium-sensing receptor (CaSR), a key player in the maintenance of calcium homeostasis, can influence bone modeling and remodeling by directly acting on bone cells, as demonstrated by in vivo and in vitro evidence. The modulation of CaSR signaling can play a role in bone anabolism.
Introduction
The calcium-sensing receptor (CaSR) is a key player in the maintenance of calcium homeostasis through the regulation of PTH secretion and calcium homeostasis, thus indirectly influencing bone metabolism. In addition to this role, in vitro and in vivo evidence points to direct effects of CaSR in bone modeling and remodeling. In addition, the activation of the CaSR is one of the anabolic mechanisms implicated in the action of strontium ranelate, to reduce fracture risk.
Methods
This review is based upon the acquisition of data from a PubMed enquiry using the terms “calcium sensing receptor,” “CaSR” AND “bone remodeling,” “bone modeling,” “bone turnover,” “osteoblast,” “osteoclast,” “osteocyte,” “chondrocyte,” “bone marrow,” “calcilytics,” “calcimimetics,” “strontium,” “osteoporosis,” “skeletal homeostasis,” and “bone metabolism.”
Results
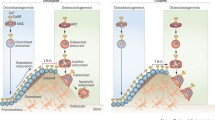
A fully functional CaSR is expressed in osteoblasts and osteoclasts, so that these cells are able to sense changes in the extracellular calcium and as a result modulate their behavior. CaSR agonists (calcimimetics) or antagonists (calcilytics) have the potential to indirectly influence skeletal homeostasis through the modulation of PTH secretion by the parathyroid glands. The bone anabolic effect of strontium ranelate, a divalent cation used as a treatment for postmenopausal and male osteoporosis, might be explained, at least in part, by the activation of CaSR in bone cells.
Conclusions
Calcium released in the bone microenvironment during remodeling is a major factor in regulating bone cells. Osteoblast and osteoclast proliferation, differentiation, and apoptosis are influenced by local extracellular calcium concentration. Thus, the calcium-sensing properties of skeletal cells can be exploited in order to modulate bone turnover and can explain the bone anabolic effects of agents developed and employed to revert osteoporosis.





Similar content being viewed by others
References
Brown EM, Gamba G, Riccardi D et al (1993) Cloning and characterization of an extracellular Ca(2+)-sensing receptor from bovine parathyroid. Nature 366:575–580
Brown EM (2000) Calcium receptor and regulation of parathyroid hormone secretion. Rev Endocr Metab Disord 1:307–315
Egbuna OI, Brown EM (2008) Hypercalcaemic and hypocalcaemic conditions due to calcium-sensing receptor mutations. Best Pract Res Clin Rheumatol 22:129–148
Loupy A, Ramakrishnan SK, Wootla B et al (2012) PTH-independent regulation of blood calcium concentration by the calcium-sensing receptor. J Clin Invest 122:3355–3367
Brown EM, Chattopadhyay N, Yano S (2004) Calcium-sensing receptors in bone cells. J Musculoskelet Neuronal Interact 4:412–413
Martin TJ, Seeman E (2008) Bone remodelling: its local regulation and the emergence of bone fragility. Best Pract Res Clin Endocrinol Metab 22:701–722
Raisz LG (2005) Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J Clin Invest 115:3318–3325
Marie PJ (2010) The calcium-sensing receptor in bone cells: a potential therapeutic target in osteoporosis. Bone 46:571–576
Ward DT, Riccardi D (2012) New concepts in calcium-sensing receptor pharmacology and signalling. Br J Pharmacol 165:35–48
Chakravarti B, Chattopadhyay N, Brown EM (2012) Signaling through the extracellular calcium-sensing receptor (CaSR). Adv Exp Med Biol 740:103–142
Dvorak MM, Riccardi D (2004) Ca2+ as an extracellular signal in bone. Cell Calcium 35:249–255
Parfitt AM (1989) Plasma calcium control at quiescent bone surfaces: a new approach to the homeostatic function of bone lining cells. Bone 10:87–88
Ye CP, Yamaguchi T, Chattopadhyay N et al (2000) Extracellular calcium-sensing-receptor (CaR)-mediated opening of an outward K(+) channel in murine MC3T3-E1 osteoblastic cells: evidence for expression of a functional CaR. Bone 27:21–27
Huang Z, Cheng SL, Slatopolsky E (2001) Sustained activation of the extracellular signal-regulated kinase pathway is required for extracellular calcium stimulation of human osteoblast proliferation. J Biol Chem 276:21351–21358
Dvorak MM, Siddiqua A, Ward DT et al (2004) Physiological changes in extracellular calcium concentration directly control osteoblast function in the absence of calciotropic hormones. Proc Natl Acad Sci U S A 101:5140–5145
Berger CE, Rathod H, Gillespie JI et al (2001) Scanning electrochemical microscopy at the surface of bone-resorbing osteoclasts: evidence for steady-state disposal and intracellular functional compartmentalization of calcium. J Bone Miner Res 16:2092–2102
Raisz LG, Niemann I (1969) Effect of phosphate, calcium and magnesium on bone resorption and hormonal responses in tissue culture. Endocrinology 85:446–452
Brown EM, Lian JB (2008) New insights in bone biology: unmasking skeletal effects of the extracellular calcium-sensing receptor. Sci Signal 1(35):pe40. doi:10.1126/scisignal.135pe40
House MG, Kohlmeier L, Chattopadhyay N et al (1997) Expression of an extracellular calcium-sensing receptor in human and mouse bone marrow cells. J Bone Miner Res 12:1959–1970
Yamaguchi T, Chattopadhyay N, Kifor O, Brown EM (1998) Extracellular calcium (Ca2+(o))-sensing receptor in a murine bone marrow-derived stromal cell line (ST2): potential mediator of the actions of Ca2+(o) on the function of ST2 cells. Endocrinology 139:3561–3568
Pi M, Hinson TK, Quarles LD (1999) Failure to detect the extracellular calcium-sensing receptor (CasR) in human osteoblast cell lines. J Bone Miner Res 14:1310–1319
Yamaguchi T, Kifor O, Chattopadhyay N, Brown EM (1998) Expression of extracellular calcium (Ca2 + o)-sensing receptor in the clonal osteoblast-like cell lines, UMR-106 and SAOS-2. Biochem Biophys Res Commun 243:753–757
Yamaguchi T, Chattopadhyay N, Kifor O et al (1998) Mouse osteoblastic cell line (MC3T3-E1) expresses extracellular calcium (Ca2+o)-sensing receptor and its agonists stimulate chemotaxis and proliferation of MC3T3-E1 cells. J Bone Miner Res 13:1530–1538
Yamaguchi T, Chattopadhyay N, Kifor O et al (2001) Expression of extracellular calcium-sensing receptor in human osteoblastic MG-63 cell line. Am J Physiol Cell Physiol 280:C382–C393
Yamaguchi T, Olozak I, Chattopadhyay N et al (1998) Expression of extracellular calcium (Ca2+o)-sensing receptor in human peripheral blood monocytes. Biochem Biophys Res Commun 246:501–506
Kameda T, Mano H, Yamada Y et al (1998) Calcium-sensing receptor in mature osteoclasts, which are bone resorbing cells. Biochem Biophys Res Commun 245:419–422
Chang W, Tu C, Chen TH et al (1999) Expression and signal transduction of calcium-sensing receptors in cartilage and bone. Endocrinology 140:5883–5893
Kamioka H, Miki Y, Sumitani K et al (1995) Extracellular calcium causes the release of calcium from intracellular stores in chick osteocytes. Biochem Biophys Res Commun 212:692–696
Godwin SL, Soltoff SP (1997) Extracellular calcium and platelet-derived growth factor promote receptor-mediated chemotaxis in osteoblasts through different signaling pathways. J Biol Chem 272:11307–11312
Nakade O, Takahashi K, Takuma T et al (2001) Effect of extracellular calcium on the gene expression of bone morphogenetic protein-2 and -4 of normal human bone cells. J Bone Miner Metab 19:13–19
Yamauchi M, Yamaguchi T, Kaji H et al (2005) Involvement of calcium-sensing receptor in osteoblastic differentiation of mouse MC3T3-E1 cells. Am J Physiol Endocrinol Metab 288:E608–E616
Deyama A, Deyama Y, Matsumoto A et al (1999) A low calcium environment enhances AP-1 transcription factor-mediated gene expression in the development of osteoblastic MC3T3-E1 cells. Miner Electrolyte Metab 25:147–160
Godwin SL, Soltoff SP (2002) Calcium-sensing receptor-mediated activation of phospholipase C-gamma1 is downstream of phospholipase C-beta and protein kinase C in MC3T3-E1 osteoblasts. Bone 30:559–566
Chattopadhyay N, Yano S, Tfelt-Hansen J et al (2004) Mitogenic action of calcium-sensing receptor on rat calvarial osteoblasts. Endocrinology 145:3451–3462
Rybchyn MS, Slater M, Conigrave AD, Mason RS (2011) An Akt-dependent increase in canonical Wnt signaling and a decrease in sclerostin protein levels are involved in strontium ranelate-induced osteogenic effects in human osteoblasts. J Biol Chem 286:23771–23779
Hu F, Pan L, Zhang K et al (2014) Elevation of extracellular Ca2+ induces store-operated calcium entry via calcium-sensing receptors: a pathway contributes to the proliferation of osteoblasts. PLoS One 9:e107217
Koori K, Maeda H, Fujii S et al (2014) The roles of calcium-sensing receptor and calcium channel in osteogenic differentiation of undifferentiated periodontal ligament cells. Cell Tissue Res 357:707–718
Bennett BD, Alvarez U, Hruska KA (2001) Receptor-operated osteoclast calcium sensing. Endocrinology 142:1968–1974
Kanatani M, Sugimoto T, Kanzawa M et al (1999) High extracellular calcium inhibits osteoclast-like cell formation by directly acting on the calcium-sensing receptor existing in osteoclast precursor cells. Biochem Biophys Res Commun 261:144–148
Lorget F, Kamel S, Mentaverri R et al (2000) High extracellular calcium concentrations directly stimulate osteoclast apoptosis. Biochem Biophys Res Commun 268:899–903
Takeyama S, Yoshimura Y, Shirai Y et al (2000) Low calcium environment effects osteoprotegerin ligand/osteoclast differentiation factor. Biochem Biophys Res Commun 276:524–529
Mentaverri R, Yano S, Chattopadhyay N et al (2006) The calcium sensing receptor is directly involved in both osteoclast differentiation and apoptosis. FASEB J 20:2562–2564
Boudot C, Saidak Z, Boulanouar AK et al (2010) Implication of the calcium sensing receptor and the phosphoinositide 3-kinase/Akt pathway in the extracellular calcium-mediated migration of RAW 264.7 osteoclast precursor cells. Bone 46:1416–1423
Bonen DK, Schmid TM (1991) Elevated extracellular calcium concentrations induce type X collagen synthesis in chondrocyte cultures. J Cell Biol 115:1171–1178
Jacenko O, Tuan RS (1995) Chondrogenic potential of chick embryonic calvaria: I. Low calcium permits cartilage differentiation. Dev Dyn 202:13–26
Wang D, Canaff L, Davidson D et al (2001) Alterations in the sensing and transport of phosphate and calcium by differentiating chondrocytes. J Biol Chem 276:33995–34005
Chang W, Tu C, Pratt S et al (2002) Extracellular Ca(2+)-sensing receptors modulate matrix production and mineralization in chondrogenic RCJ3.1C5.18 cells. Endocrinology 143:1467–1474
Wu S, Palese T, Mishra OP et al (2004) Effects of Ca2+ sensing receptor activation in the growth plate. FASEB J 18:143–145
Ho C, Conner DA, Pollak MR et al (1995) A mouse model of human familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism. Nat Genet 11:389–394
Garner SC, Pi M, Tu Q, Quarles LD (2001) Rickets in cation-sensing receptor-deficient mice: an unexpected skeletal phenotype. Endocrinology 142:3996–4005
Tu Q, Pi M, Karsenty G et al (2003) Rescue of the skeletal phenotype in CasR-deficient mice by transfer onto the Gcm2 null background. J Clin Invest 111:1029–1037
Kos CH, Karaplis AC, Peng JB et al (2003) The calcium-sensing receptor is required for normal calcium homeostasis independent of parathyroid hormone. J Clin Invest 111:1021–1028
Liu J, Lv F, Sun W et al (2011) The abnormal phenotypes of cartilage and bone in calcium-sensing receptor deficient mice are dependent on the actions of calcium, phosphorus, and PTH. PLoS Genet 7:e1002294
Oda Y, Tu CL, Pillai S, Bikle DD (1998) The calcium sensing receptor and its alternatively spliced form in keratinocyte differentiation. J Biol Chem 273:23344–23352
Rodriguez L, Tu C, Cheng Z et al (2005) Expression and functional assessment of an alternatively spliced extracellular Ca2+-sensing receptor in growth plate chondrocytes. Endocrinology 146:5294–5303
Hough TA, Bogani D, Cheeseman MT et al (2004) Activating calcium-sensing receptor mutation in the mouse is associated with cataracts and ectopic calcification. Proc Natl Acad Sci U S A 101:13566–13571
Dvorak MM, Chen TH, Orwoll B et al (2007) Constitutive activity of the osteoblast Ca2+-sensing receptor promotes loss of cancellous bone. Endocrinology 148:3156–3163
Chang W, Dvorak M, Shoback D (2010) Assessing constitutive activity of extracellular calcium-sensing receptors in vitro and in bone. Methods Enzymol 484:253–266
Chang W, Tu C, Chen TH et al (2008) The extracellular calcium-sensing receptor (CaSR) is a critical modulator of skeletal development. Sci Signal 1(35):ra1. doi:10.1126/scisignal.1159945
Liu F, Woitge HW, Braut A et al (2004) Expression and activity of osteoblast-targeted Cre recombinase transgenes in murine skeletal tissues. Int J Dev Biol 48:645–653
Dvorak-Ewell MM, Chen TH, Liang N et al (2011) Osteoblast extracellular Ca2+-sensing receptor regulates bone development, mineralization, and turnover. J Bone Miner Res 26:2935–2947
Richard C, Huo R, Samadfam R et al (2010) The calcium-sensing receptor and 25-hydroxyvitamin D-1alpha-hydroxylase interact to modulate skeletal growth and bone turnover. J Bone Miner Res 25:1627–1636
Sun W, Sun W, Liu J et al (2010) Alterations in phosphorus, calcium and PTHrP contribute to defects in dental and dental alveolar bone formation in calcium-sensing receptor-deficient mice. Development 137:985–992
Shu L, Ji J, Zhu Q et al (2011) The calcium-sensing receptor mediates bone turnover induced by dietary calcium and parathyroid hormone in neonates. J Bone Miner Res 26:1057–1071
Xue Y, Xiao Y, Liu J et al (2012) The calcium-sensing receptor complements parathyroid hormone-induced bone turnover in discrete skeletal compartments in mice. Am J Physiol Endocrinol Metab 302:E841–E851
Blair HC, Schlesinger PH, Huang CL, Zaidi M (2007) Calcium signalling and calcium transport in bone disease. Subcell Biochem 45:539–562
Zaidi M, Shankar VS, Tunwell R et al (1995) A ryanodine receptor-like molecule expressed in the osteoclast plasma membrane functions in extracellular Ca2+ sensing. J Clin Invest 96:1582–1590
Tu Q, Pi M, Quarles LD (2003) Calcyclin mediates serum response element (SRE) activation by an osteoblastic extracellular cation-sensing mechanism. J Bone Miner Res 18:1825–1833
Pi M, Quarles LD (2004) A novel cation-sensing mechanism in osteoblasts is a molecular target for strontium. J Bone Miner Res 19:862–869
Pi M, Faber P, Ekema G et al (2005) Identification of a novel extracellular cation-sensing G-protein-coupled receptor. J Biol Chem 280:40201–40209
Clemmensen C, Smajilovic S, Wellendorph P, Bräuner-Osborne H (2014) The GPCR, class C, group 6, subtype A (GPRC6A) receptor: from cloning to physiological function. Br J Pharmacol 171:1129–1141
Pi M, Chen L, Huang MZ et al (2008) GPRC6A null mice exhibit osteopenia, feminization and metabolic syndrome. PLoS One 3:e3858
Oury F, Ferron M, Huizhen W et al (2013) Osteocalcin regulates murine and human fertility through a pancreas-bone-testis axis. J Clin Invest 123:2421–2433
Wellendorph P, Johansen LD, Jensen AA et al (2009) No evidence for a bone phenotype in GPRC6A knockout mice under normal physiological conditions. J Mol Endocrinol 42:215–223
Barradas AM, Fernandes HA, Groen N et al (2012) A calcium-induced signaling cascade leading to osteogenic differentiation of human bone marrow-derived mesenchymal stromal cells. Biomaterials 33:3205–3215
Khoshniat S, Bourgine A, Julien M et al (2011) Phosphate-dependent stimulation of MGP and OPN expression in osteoblasts via the ERK1/2 pathway is modulated by calcium. Bone 48:894–902
Law WM Jr, Wahner HW, Heath H 3rd (1984) Bone mineral density and skeletal fractures in familial benign hypercalcemia (hypocalciuric hypercalcemia). Mayo Clin Proc 59:811–815
Kristiansen JH, Rødbro P, Christiansen C et al (1987) Familial hypocalciuric hypercalcaemia. III: bone mineral metabolism. Clin Endocrinol (Oxf) 26:713–716
Abugassa S, Nordenström J, Järhult J (1992) Bone mineral density in patients with familial hypocalciuric hypercalcaemia (FHH). Eur J Surg 158:397–402
Christensen SE, Nissen PH, Vestergaard P et al (2009) Skeletal consequences of familial hypocalciuric hypercalcaemia vs. primary hyperparathyroidism. Clin Endocrinol (Oxf) 71:798–807
Theman TA, Collins MT, Dempster DW et al (2009) PTH(1-34) replacement therapy in a child with hypoparathyroidism caused by a sporadic calcium receptor mutation. J Bone Miner Res 24:964–973
Jakobsen NF, Rolighed L, Moser E et al (2014) Increased trabecular volumetric bone mass density in familial hypocalciuric hypercalcemia (FHH) type 1: a cross-sectional study. Calcif Tissue Int 95:141–152
Nemeth EF, Shoback D (2013) Calcimimetic and calcilytic drugs for treating bone and mineral-related disorders. Best Pract Res Clin Endocrinol Metab 27:373–384
Cianferotti L, D’Asta F, Brandi ML (2013) A review on strontium ranelate long-term antifracture efficacy in the treatment of postmenopausal osteoporosis. Ther Adv Musculoskelet Dis 5:127–139
Leach K, Conigrave AD, Sexton PM, Christopoulos A (2015) Towards tissue-specific pharmacology: insights from the calcium-sensing receptor as a paradigm for GPCR (patho)physiological bias. Trends Pharmacol Sci 36:215–225
Nemeth EF, Delmar EG, Heaton WL et al (2001) Calcilytic compounds: potent and selective Ca2+ receptor antagonists that stimulate secretion of parathyroid hormone. J Pharmacol Exp Ther 299:323–331
Rubin MR, Bilezikian JP (2005) Parathyroid hormone as an anabolic skeletal therapy. Drugs 65:2481–2498
Toulis KA, Anastasilakis AD, Polyzos SA, Makras P (2011) Targeting the osteoblast: approved and experimental anabolic agents for the treatment of osteoporosis. Hormones (Athens) 10:174–195
Riccardi D, Kemp PJ (2012) The calcium-sensing receptor beyond extracellular calcium homeostasis: conception, development, adult physiology, and disease. Annu Rev Physiol 74:271–297
Fitzpatrick LA, Dabrowski CE, Cicconetti G et al (2011) The effects of ronacaleret, a calcium-sensing receptor antagonist, on bone mineral density and biochemical markers of bone turnover in postmenopausal women with low bone mineral density. J Clin Endocrinol Metab 96:2441–2449
Fitzpatrick LA, Smith PL, McBride TA et al (2011) Ronacaleret, a calcium-sensing receptor antagonist, has no significant effect on radial fracture healing time: results of a randomized, double-blinded, placebo-controlled Phase II clinical trial. Bone 49:845–852
Fitzpatrick LA, Dabrowski CE, Cicconetti G et al (2012) Ronacaleret, a calcium-sensing receptor antagonist, increases trabecular but not cortical bone in postmenopausal women. J Bone Miner Res 27:255–262
Caltabiano S, Dollery CT, Hossain M et al (2013) Characterization of the effect of chronic administration of a calcium-sensing receptor antagonist, ronacaleret, on renal calcium excretion and serum calcium in postmenopausal women. Bone 56:154–162
Dempster DW, Müller R, Zhou H et al (2007) Preserved three-dimensional cancellous bone structure in mild primary hyperparathyroidism. Bone 41:19–24
Fitzpatrick LA, Wooddell M, Dabrowski CE et al (2014) Bone mineral density changes following discontinuation of ronacaleret treatment: off-treatment extension of a randomized, dose-finding phase II trial. Bone 67:104–108
Shinagawa Y, Inoue T, Katsushima T et al (2010) Discovery of a potent and short-acting oral calcilytic with a pulsatile secretion of parathyroid hormone. ACS Med Chem Lett 2:238–242
Kimura S, Nakagawa T, Matsuo Y et al (2011) JTT-305, an orally active calcium-sensing receptor antagonist, stimulates transient parathyroid hormone release and bone formation in ovariectomized rats. Eur J Pharmacol 668:331–336
Fukumoto S, Nakamura T, Nishizawa Y et al (2009) Randomized, single‐blinded placebo‐controlled study of a novel calcilytic, JTT‐305, in patients with postmenopausal osteoporosis. J Bone Miner Res 24(Suppl 1):S40
Halse J, Greenspan S, Cosman F et al (2014) A phase 2, randomized, placebo-controlled, dose-ranging study of the calcium-sensing receptor antagonist MK-5442 in the treatment of postmenopausal women with osteoporosis. J Clin Endocrinol Metab 99(11):E2207–E2215
John MR, Widler L, Gamse R et al (2011) ATF936, a novel oral calcilytic, increases bone mineral density in rats and transiently releases parathyroid hormone in humans. Bone 49:233–241
John MR, Harfst E, Loeffler J et al (2014) AXT914 a novel, orally-active parathyroid hormone-releasing drug in two early studies of healthy volunteers and postmenopausal women. Bone 64:204–210
Widler L (2011) Calcilytics: antagonists of the calcium-sensing receptor for the treatment of osteoporosis. Future Med Chem 3:535–547
Letz S, Rus R, Haag C et al (2010) Novel activating mutations of the calcium-sensing receptor: the calcilytic NPS-2143 mitigates excessive signal transduction of mutant receptors. J Clin Endocrinol Metab 95:E229–E233
Park SY, Mun HC, Eom YS et al (2013) Identification and characterization of D410E, a novel mutation in the loop 3 domain of CASR, in autosomal dominant hypocalcemia and a therapeutic approach using a novel calcilytic, AXT914. Clin Endocrinol (Oxf) 78:687–693
Nemeth EF, Steffey ME, Hammerland LG et al (1998) Calcimimetics with potent and selective activity on the parathyroid calcium receptor. Proc Natl Acad Sci U S A 95:4040–4045
Brown EM (2010) Clinical utility of calcimimetics targeting the extracellular calcium-sensing receptor (CaSR). Biochem Pharmacol 80:297–307
Peacock M, Bilezikian JP, Klassen PS et al (2005) Cinacalcet hydrochloride maintains long-term normocalcemia in patients with primary hyperparathyroidism. J Clin Endocrinol Metab 90:135–141
Peacock M, Bolognese MA, Borofsky M et al (2009) Cinacalcet treatment of primary hyperparathyroidism: biochemical and bone densitometric outcomes in a five-year study. J Clin Endocrinol Metab 94:4860–4867
Strippoli GF, Tong A, Palmer SC et al (2006) Calcimimetics for secondary hyperparathyroidism in chronic kidney disease patients. Cochrane Database Syst Rev 4:CD006254
Nemeth EF (2010) Calcimimetics and calcilytics in the treatment of chronic kidney disease-mineral bone disorder. In: Olgaard K, Salusky IB, Silver J (eds) The spectrum of mineral and bone disorders in chronic kidney disease, 2nd edn. Oxford University Press, Oxford, pp 443–461
Behets GJ, Spasovski G, Sterling LR et al (2014) Bone histomorphometry before and after long-term treatment with cinacalcet in dialysis patients with secondary hyperparathyroidism. Kidney Int. doi:10.1038/ki.2014.349
Reginster JY, Neuprez A, Dardenne N et al (2014) Efficacy and safety of currently marketed anti-osteoporosis medications. Best Pract Res Clin Endocrinol Metab 28:809–834
Marie PJ (2006) Strontium ranelate: a dual mode of action rebalancing bone turnover in favour of bone formation. Curr Opin Rheumatol 18(Suppl 1):S11–S15
Baron R, Tsouderos Y (2002) In vitro effects of S12911-2 on osteoclast function and bone marrow macrophage differentiation. Eur J Pharmacol 450:11–17
Ammann P, Shen V, Robin B et al (2004) Strontium ranelate improves bone resistance by increasing bone mass and improving architecture in intact female rats. J Bone Miner Res 19:2012–2020
Meunier PJ, Roux C, Seeman E et al (2004) The effects of strontium ranelate on the risk of vertebral fracture in women with postmenopausal osteoporosis. N Engl J Med 350:459–468
Reginster JY, Seeman E, De Vernejoul MC et al (2005) Strontium ranelate reduces the risk of nonvertebral fractures in postmenopausal women with osteoporosis: Treatment of Peripheral Osteoporosis (TROPOS) study. J Clin Endocrinol Metab 90:2816–2822
Saidak Z, Marie PJ (2012) Strontium signaling: molecular mechanisms and therapeutic implications in osteoporosis. Pharmacol Ther 136:216–226
Caudrillier A, Hurtel-Lemaire AS, Wattel A et al (2010) Strontium ranelate decreases receptor activator of nuclear factor-ΚB ligand-induced osteoclastic differentiation in vitro: involvement of the calcium-sensing receptor. Mol Pharmacol 78:569–576
Hurtel-Lemaire AS, Mentaverri R, Caudrillier A et al (2009) The calcium-sensing receptor is involved in strontium ranelate-induced osteoclast apoptosis. New insights into the associated signaling pathways. J Biol Chem 284:575–584
Fromigué O, Haÿ E, Barbara A et al (2009) Calcium sensing receptor-dependent and receptor-independent activation of osteoblast replication and survival by strontium ranelate. J Cell Mol Med 13:2189–2199
Brennan TC, Rybchyn MS, Green W et al (2009) Osteoblasts play key roles in the mechanisms of action of strontium ranelate. Br J Pharmacol 157:1291–1300
Conflict of interest
Luisella Cianferotti, Ana Rita Gomes, Sergio Fabbri, and Annalisa Tanini declare that they have no conflict of interest. Maria Luisa Brandi declares that she has conflict of interest as consultant and recipient of grants from Amgen, Eli Lilly, MSD, Novartis, Roche, and Servier.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cianferotti, L., Gomes, A.R., Fabbri, S. et al. The calcium-sensing receptor in bone metabolism: from bench to bedside and back. Osteoporos Int 26, 2055–2071 (2015). https://doi.org/10.1007/s00198-015-3203-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-015-3203-1