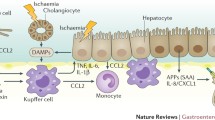
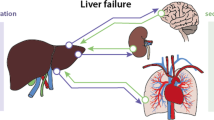
Abstract
Objective
In ICU patients, abnormal liver tests are common. Markers of cholestasis are associated with adverse outcome. Research has focused on the possibility that mild hyperbilirubinemia, instead of indicating inadvertent cholestasis, may be adaptive and beneficial. These new insights are reviewed and integrated in the state-of-the-art knowledge on hepatobiliary alterations during sepsis and other critical illnesses.
Data sources
Relevant publications were searched in Medline with search terms bile, bile acids, cholestasis, critical illness, intensive care, sepsis, alone or in combination.
Data synthesis
Studies have shown that bilirubin, but also bile acids, the main active constitutes of bile, are increased in plasma of patients with critical illnesses. In particular the conjugated fractions of bilirubin and bile acids are high, indicating that during critical illness the liver is capable of converting these molecules to less toxic forms. In human liver biopsies of prolonged critically ill patients, expression of bile acid excretion pumps towards the bile canaliculi was lower, while alternative transporters towards the systemic circulation were upregulated. Remarkably, in the presence of increased circulating bile acids, expression of enzymes controlling synthesis of bile acids was not suppressed. This suggested loss of feedback inhibition of bile acids synthesis, possibly explained by the observed cytoplasmic retention of the nuclear FXR/RXR heterodimer. As macronutrient restriction during acute critical illness, an intervention that improved outcome, was found to further increase plasma bilirubin while reducing other markers of cholestasis, a potentially protective role of hyperbilirubinemia was suggested.
Conclusion
The increase in circulating levels of conjugated bile acids and bilirubin in response to acute sepsis/critical illnesses may not necessarily point to cholestasis as a pathophysiological entity. Instead it may be the result of an adaptively altered bile acid production and transport back towards the systemic circulation. How these changes could be beneficial for survival should be further investigated.




Similar content being viewed by others
References
Nesseler N, Launey Y, Aninat C, Morel F, Mallédant Y, Seguin P (2012) Clinical review: the liver in sepsis. Crit Care 16:235
Kramer L, Jordan B, Druml W, Bauer P, Metnitz PGH, Austrian Epidemiologic Study on Intensive Care ASDI Study Group (2007) Incidence and prognosis of early hepatic dysfunction in critically ill patients—a prospective multicenter study. Crit Care Med 35:1099–1104
Fuhrmann V, Kneidinger N, Herkner H, Heinz G, Nikfardjam M, Bojic A, Schellongowski P, Angermayr B, Schöniger-Hekele M, Madl C, Schenk P (2011) Impact of hypoxic hepatitis on mortality in the intensive care unit. Intensive Care Med 37:1302–1310
Thomson SJ, Cowan ML, Johnston I, Musa S, Grounds M, Rahman TM (2009) ‘Liver function tests’ on the intensive care unit: a prospective, observational study. Intensive Care Med 35:1406–1411
Horvatits T, Trauner M, Fuhrmann V (2013) Hypoxic liver injury and cholestasis in critically ill patients. Curr Opin Crit Care 19:128–132
Henrion J (2012) Hypoxic hepatitis. Liver Int 32:1039–1052
Brienza N, Dalfino L, Cinnella G, Diele C, Bruno F, Fiore T (2006) Jaundice in critical illness: promoting factors of a concealed reality. Intensive Care Med 32:267–274
Harbrecht BG, Zenati MS, Doyle HR, McMichael J, Townsend RN, Clancy KD, Peitzman AB (2002) Hepatic dysfunction increases length of stay and risk of death after injury. J Trauma 53:517–523
Mesotten D, Wauters J, Van den Berghe G, Wouters PJ, Milants I, Wilmer A (2009) The effect of strict blood glucose control on biliary sludge and cholestasis in critically ill patients. J Clin Endocrinol Metab 94:2345–2352
van Deursen VM, Damman K, Hillege HL, van Beek AP, van Veldhuisen DJ, Voors AA (2010) Abnormal liver function in relation to hemodynamic profile in heart failure patients. J Card Fail 16:84–90
Boland GW, Slater G, Lu DS, Eisenberg P, Lee MJ, Mueller PR (2000) Prevalence and significance of gallbladder abnormalities seen on sonography in intensive care unit patients. AJR Am J Roentgenol 174:973–977
Murray FE, Stinchcombe SJ, Hawkey CJ (1992) Development of biliary sludge in patients on intensive care unit: results of a prospective ultrasonographic study. Gut 33:1123–1125
Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, Reinhart CK, Suter PM, Thijs LG (1996) The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 22:707–710
Trauner M, Fickert P, Stauber RE (1999) Inflammation-induced cholestasis. J Gastroenterol Hepatol 14:946–959
Grau T, Bonet A, Rubio M, Mateo D, Farré M, Acosta JA, Blesa A, Montejo JC, de Lorenzo AG, Mesejo A, Working Group on Nutrition and Metabolism of the Spanish Society of Critical Care (2007) Liver dysfunction associated with artificial nutrition in critically ill patients. Crit Care 11:R10
Lat I, Foster DR, Erstad B (2010) Drug-induced acute liver failure and gastrointestinal complications. Crit Care Med 38:S175–S187
Porez G, Prawitt J, Gross B, Staels B (2012) Bile acid receptors as targets for the treatment of dyslipidemia and cardiovascular disease. J Lipid Res 53:1723–1737
Chiang JYL (2009) Bile acids: regulation of synthesis. J Lipid Res 50:1955–1966
Kubitz R, Droge C, Stindt J, Weissenberger K, Haussinger D (2012) The bile salt export pump (BSEP) in health and disease. Clin Res Hepatol Gastroenterol 36:536–553
Alrefai WA, Gill RK (2007) Bile acid transporters: structure, function, regulation and pathophysiological implications. Pharm Res 24:1803–1823
Soroka CJ, Lee JM, Azzaroli F, Boyer JL (2001) Cellular localization and up-regulation of multidrug resistance-associated protein 3 in hepatocytes and cholangiocytes during obstructive cholestasis in rat liver. Hepatology 33:783–791
Denk GU, Soroka CJ, Takeyama Y, Chen W-S, Schuetz JD, Boyer JL (2004) Multidrug resistance-associated protein 4 is up-regulated in liver but down-regulated in kidney in obstructive cholestasis in the rat. J Hepatol 40:585–591
Lee J, Azzaroli F, Wang L, Soroka CJ, Gigliozzi A, Setchell KD, Kramer W, Boyer JL (2001) Adaptive regulation of bile salt transporters in kidney and liver in obstructive cholestasis in the rat. Gastroenterology 121:1473–1484
Glass CK, Ogawa S (2006) Combinatorial roles of nuclear receptors in inflammation and immunity. Nat Rev Immunol 6:44–55
Goodwin B, Jones SA, Price RR, Watson MA, McKee DD, Moore LB, Galardi C, Wilson JG, Lewis MC, Roth ME, Maloney PR, Willson TM, Kliewer SA (2000) A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol Cell 6:517–526
Lu TT, Makishima M, Repa JJ, Schoonjans K, Kerr TA, Auwerx J, Mangelsdorf DJ (2000) Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors. Mol Cell 6:507–515
Tirona RG, Kim RB (2005) Nuclear receptors and drug disposition gene regulation. J Pharm Sci 94:1169–1186
Chiang JYL (2003) Bile acid regulation of hepatic physiology: III. Bile acids and nuclear receptors. Am J Physiol Gastrointest Liver Physiol 284:G349–G356
Halilbasic E, Baghdasaryan A, Trauner M (2013) Nuclear receptors as drug targets in cholestatic liver diseases. Clin Liver Dis 17:161–189
Glicksman C, Pournaras DJ, Wright M, Roberts R, Mahon D, Welbourn R, Sherwood R, Alaghband-Zadeh J, le Roux CW (2010) Postprandial plasma bile acid responses in normal weight and obese subjects. Ann Clin Biochem 47:482–484
Ma K, Xiao R, Tseng H-T, Shan L, Fu L, Moore DD (2009) Circadian dysregulation disrupts bile acid homeostasis. PLoS One 4:e6843
Thomas C, Pellicciari R, Pruzanski M, Auwerx J, Schoonjans K (2008) Targeting bile-acid signalling for metabolic diseases. Nat Rev Drug Discov 7:678–693
Houten SM, Watanabe M, Auwerx J (2006) Endocrine functions of bile acids. EMBO J 25:1419–1425
Schaap FG, Trauner M, Jansen PLM (2014) Bile acid receptors as targets for drug development. Nat Rev Gastroenterol Hepatol 11:55–67
de Aguiar Vallim TQ, Tarling EJ, Edwards PA (2013) Pleiotropic roles of bile acids in metabolism. Cell Metab 17:657–669
Keitel V, Kubitz R, Haussinger D (2008) Endocrine and paracrine role of bile acids. World J Gastroenterol 14:5620–5629
Hirata K, Ikeda S, Honma T, Mitaka T, Furuhata T, Katsuramaki T, Hata F, Mukaiya M (2001) Sepsis and cholestasis: basic findings in the sinusoid and bile canaliculus. J Hepatobiliary Pancreatic Surg 8:20–26
Koskinas J, Gomatos IP, Tiniakos DG, Memos N, Boutsikou M, Garatzioti A, Archimandritis A, Betrosian A (2008) Liver histology in ICU patients dying from sepsis: a clinico-pathological study. World J Gastroenterol 14:1389–1393
Lysova NL, Gurevich LE, Trusov OA, Shchegolev AI, Mishnev OD (2001) Immunohistochemical characteristics of the liver in patients with peritonitis (early autopsy). Bull Exp Biol Med 132:1125–1129
Lefkowitch JH (1982) Bile ductular cholestasis: an ominous histopathologic sign related to sepsis and “cholangitis lenta”. Hum Pathol 13:19–24
Trauner M, Meier PJ, Boyer JL (1998) Molecular pathogenesis of cholestasis. New Engl J Med 339:1217–1227
Jaeger C, Mayer G, Henrich R, Gossner L, Rabenstein T, May A, Guenter E, Ell C (2006) Secondary sclerosing cholangitis after long-term treatment in an intensive care unit: clinical presentation, endoscopic findings, treatment, and follow-up. Endoscopy 38:730–734
Kulaksiz H, Heuberger D, Engler S, Stiehl A (2008) Poor outcome in progressive sclerosing cholangitis after septic shock. Endoscopy 40:214–218
Green RM, Beier D, Gollan JL (1996) Regulation of hepatocyte bile salt transporters by endotoxin and inflammatory cytokines in rodents. Gastroenterology 111:193–198
Vanwijngaerden YM, Wauters J, Langouche L, Vander Perre S, Liddle C, Coulter S, Vanderborght S, Roskams T, Wilmer A, Van den Berghe G, Mesotten D (2011) Critical illness evokes elevated circulating bile acids related to altered hepatic transporter and nuclear receptor expression. Hepatology 54:1741–1752
Andrejko KM, Raj NR, Kim PK, Cereda M, Deutschman CS (2008) IL-6 modulates sepsis-induced decreases in transcription of hepatic organic anion and bile acid transporters. Shock 29:490–496
Geier A, Dietrich CG, Voigt S, Ananthanarayanan M, Lammert F, Schmitz A, Trauner M, Wasmuth HE, Boraschi D, Balasubramaniyan N, Suchy FJ, Matern S, Gartung C (2005) Cytokine-dependent regulation of hepatic organic anion transporter gene transactivators in mouse liver. Am J Physiol Gastrointest Liver Physiol 289:G831–G841
Cherrington NJ, Slitt AL, Li N, Klaassen CD (2004) Lipopolysaccharide-mediated regulation of hepatic transporter mRNA levels in rats. Drug Metab Dispos 32:734–741
Elferink MGL, Olinga P, Draaisma AL, Merema MT, Faber KN, Slooff MJH, Meijer DKF, Groothuis GMM (2004) LPS-induced downregulation of MRP2 and BSEP in human liver is due to a posttranscriptional process. Am J Physiol Gastrointest Liver Physiol 287:G1008–G1016
Donner MG, Warskulat U, Saha N, Häussinger D (2004) Enhanced expression of basolateral multidrug resistance protein isoforms Mrp3 and Mrp5 in rat liver by LPS. Biol Chem 385:331–339
Vanwijngaerden Y-M, Langouche L, Derde S, Liddle C, Coulter S, Van den Berghe G, Mesotten D (2014) Impact of parenteral nutrition versus fasting on hepatic bile acid production and transport in a rabbit model of prolonged critical illness. Shock 41:48–54
Zimmerman TL, Thevananther S, Ghose R, Burns AR, Karpen SJ (2006) Nuclear export of retinoid X receptor alpha in response to interleukin-1beta-mediated cell signaling: roles for JNK and SER260. J Biol Chem 281:15434–15440
Geier A, Fickert P, Trauner M (2006) Mechanisms of disease: mechanisms and clinical implications of cholestasis in sepsis. Nat Clin Pract Gastroenterol Hepatol 3:574–585
Whitehead MW, Hainsworth I, Kingham JG (2001) The causes of obvious jaundice in South West Wales: perceptions versus reality. Gut 48:409–413
Clements WD, Parks R, Erwin P, Halliday MI, Barr J, Rowlands BJ (1996) Role of the gut in the pathophysiology of extrahepatic biliary obstruction. Gut 39:587–593
te Boekhorst T, Urlus M, Doesburg W, Yap SH, Goris RJ (1988) Etiologic factors of jaundice in severely ill patients. A retrospective study in patients admitted to an intensive care unit with severe trauma or with septic intra-abdominal complications following surgery and without evidence of bile duct obstruction. J Hepatol 7:111–117
Carter BA, Shulman RJ (2007) Mechanisms of disease: update on the molecular etiology and fundamentals of parenteral nutrition associated cholestasis. Nat Clin Pract Gastroenterol Hepatol 4:277–287
Casaer MP, Mesotten D, Hermans G, Wouters PJ, Schetz M, Meyfroidt G, Van Cromphaut S, Ingels C, Meersseman P, Muller J, Vlasselaers D, Debaveye Y, Desmet L, Dubois J, Van Assche A, Vanderheyden S, Wilmer A, Van den Berghe G (2011) Early versus late parenteral nutrition in critically ill adults. New Engl J Med 365:506–517
Hermans G, Casaer MP, Clerckx B, Güiza F, Vanhullebusch T, Derde S, Meersseman P, Derese I, Mesotten D, Wouters PJ, Van Cromphaut S, Debaveye Y, Gosselink R, Gunst J, Wilmer A, Van den Berghe G, Vanhorebeek I (2013) Effect of tolerating macronutrient deficit on the development of intensive-care unit acquired weakness: a subanalysis of the EPaNIC trial. Lancet Respir Med 1:621–629
Vanwijngaerden Y-M, Langouche L, Brunner R, Debaveye Y, Gielen M, Casaer M, Liddle C, Coulter S, Wouters PJ, Wilmer A, Van den Berghe G, Mesotten D (2013) Withholding parenteral nutrition during critical illness increases plasma bilirubin but lowers the incidence of biliary sludge. Hepatology ;60:202–610
Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, Van Wijngaerden E, Bobbaers H, Bouillon R (2006) Intensive insulin therapy in the medical ICU. New Engl J Med 354:449–461
Van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R (2001) Intensive insulin therapy in critically ill patients. New Engl J Med 345:1359–1367
Gonnert FA, Recknagel P, Hilger I, Claus RA, Bauer M, Kortgen A (2013) Hepatic excretory function in sepsis: implications from biophotonic analysis of transcellular xenobiotic transport in a rodent model. Crit Care 17:R67
Recknagel P, Gonnert FA, Westermann M, Lambeck S, Lupp A, Rudiger A, Dyson A, Carré JE, Kortgen A, Krafft C, Popp J, Sponholz C, Fuhrmann V, Hilger I, Claus RA, Riedemann NC, Wetzker R, Singer M, Trauner M, Bauer M (2012) Liver dysfunction and phosphatidylinositol-3-kinase signalling in early sepsis: experimental studies in rodent models of peritonitis. PLoS Med 9:e1001338
Yang K, Köck K, Sedykh A, Tropsha A, Brouwer KLR (2013) An updated review on drug-induced cholestasis: mechanisms and investigation of physicochemical properties and pharmacokinetic parameters. J Pharm Sci 102:3037–3057
Lammert C, Bjornsson E, Niklasson A, Chalasani N (2010) Oral medications with significant hepatic metabolism at higher risk for hepatic adverse events. Hepatology 51:615–620
Maruhashi T, Soga J, Fujimura N, Idei N, Mikami S, Iwamoto Y, Kajikawa M, Matsumoto T, Kihara Y, Chayama K, Noma K, Nakashima A, Tomiyama H, Takase B, Yamashina A, Higashi Y (2012) Hyperbilirubinemia, augmentation of endothelial function, and decrease in oxidative stress in Gilbert syndrome. Circulation 126:598–603
Castilho Á, Aveleira CA, Leal EC, Simões NF, Fernandes CR, Meirinhos RI, Baptista FI, Ambrósio AF (2012) Heme oxygenase-1 protects retinal endothelial cells against high glucose- and oxidative/nitrosative stress-induced toxicity. PLoS One 7:e42428
Baranano DE, Rao M, Ferris CD, Snyder SH (2002) Biliverdin reductase: a major physiologic cytoprotectant. Proc Natl Acad Sci USA 99:16093–16098
Wang WW, Smith DLH, Zucker SD (2004) Bilirubin inhibits iNOS expression and NO production in response to endotoxin in rats. Hepatology 40:424–433
Wiesel P, Patel AP, DiFonzo N, Marria PB, Sim CU, Pellacani A, Maemura K, LeBlanc BW, Marino K, Doerschuk CM, Yet SF, Lee ME, Perrella MA (2000) Endotoxin-induced mortality is related to increased oxidative stress and end-organ dysfunction, not refractory hypotension, in heme oxygenase-1-deficient mice. Circulation 102:3015–3022
McNeilly AD, Macfarlane DP, O’Flaherty E, Livingstone DE, Mitić T, McConnell KM, McKenzie SM, Davies E, Reynolds RM, Thiesson HC, Skøtt O, Walker BR, Andrew R (2010) Bile acids modulate glucocorticoid metabolism and the hypothalamic-pituitary-adrenal axis in obstructive jaundice. J Hepatol 52:705–711
Boonen E, Vervenne H, Meersseman P, Andrew R, Mortier L, Declercq PE, Vanwijngaerden YM, Spriet I, Wouters PJ, Vander Perre S, Langouche L, Vanhorebeek I, Walker BR, Van den Berghe G (2013) Reduced cortisol metabolism during critical illness. New Engl J Med 368:1477–1488
Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H, Messaddeq N, Harney JW, Ezaki O, Kodama T, Schoonjans K, Bianco AC, Auwerx J (2006) Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature 439:484–489
Katsuma S, Hirasawa A, Tsujimoto G (2005) Bile acids promote glucagon-like peptide-1 secretion through TGR5 in a murine enteroendocrine cell line STC-1. Biochem Biophys Res Commun 329:386–390
Alsatie M, Kwo PY, Gingerich JR, Qi R, Eckert G, Cummings OW, Imperiale TF (2007) A multivariable model of clinical variables predicts advanced fibrosis in chronic hepatitis C. J Clin Gastroenterol 41:416–421
Kim I, Morimura K, Shah Y, Yang Q, Ward JM, Gonzalez FJ (2007) Spontaneous hepatocarcinogenesis in farnesoid X receptor-null mice. Carcinogenesis 28:940–946
Zhang Y, Xu P, Park K, Choi Y, Moore DD, Wang L (2008) Orphan receptor small heterodimer partner suppresses tumorigenesis by modulating cyclin D1 expression and cellular proliferation. Hepatology 48:289–298
Lang E, Gatidis S, Freise NF, Bock H, Kubitz R, Lauermann C, Orth HM, Klindt C, Schuier M, Keitel V, Reich M, Liu G, Schmidt S, Xu HC, Qadri SM, Herebian D, Pandyra AA, Mayatepek E, Gulbins E, Lang F, Haussinger D, Lang KS, Foller M, Lang PA (2015) Conjugated bilirubin triggers anemia by inducing erythrocyte death. Hepatology 61:275–284
Yang LQ, Tao KM, Liu YT, Cheung CW, Irwin MG, Wong GT, Lv H, Song JG, Wu FX, Yu WF (2011) Remifentanil preconditioning reduces hepatic ischemia-reperfusion injury in rats via inducible nitric oxide synthase expression. Anesthesiology 114:1036–1047
Zhao G, Shen X, Nan H, Yan L, Zhao H, Yu J, Lv Y (2013) Remifentanil protects liver against ischemia/reperfusion injury through activation of anti-apoptotic pathways. J Surg Res 183:827–834
Beuers U (2006) Drug insight: mechanisms and sites of action of ursodeoxycholic acid in cholestasis. Nat Clin Pract Gastroenterol Hepatol 3:318–328
Zimmerman JE, Kramer AA, McNair DS, Malila FM (2006) Acute Physiology and Chronic Health Evaluation (APACHE) IV: hospital mortality assessment for today’s critically ill patients. Crit Care Med 34:1297–1310
Le Gall JR, Lemeshow S, Saulnier F (1993) A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 270:2957–2963
Acknowledgments
Funding was provided by the Methusalem Program of the Flemish Government to GVdB via the KU Leuven University (METH/08/07); by an ERC Advanced grant (AdvG-2012-321670) to GVdB from the Ideas Program of the European Union 7th Framework Program. Senior Clinical Investigator Fellowship from the Research Foundation Flanders to DM.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no conflicts of interest to disclose.
Additional information
Take-home message: During sepsis and other critical illnesses, mild hyperbilirubinemia, instead of indicating inadvertent cholestasis, may be an adaptive and beneficial response. The observed increase in circulating levels of conjugated bile acids and bilirubin may be the result of an adaptively altered bile acid production and transport back towards the systemic circulation.
Rights and permissions
About this article
Cite this article
Jenniskens, M., Langouche, L., Vanwijngaerden, YM. et al. Cholestatic liver (dys)function during sepsis and other critical illnesses. Intensive Care Med 42, 16–27 (2016). https://doi.org/10.1007/s00134-015-4054-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-015-4054-0