Abstract
Purpose
To evaluate the hemodynamic monitoring capability and safety of a single-use miniaturized transesophageal echocardiography (TEE) probe left in place in ventilated critically ill patients.
Methods
The probe was inserted in 94 patients and designed to be left in place for up to 72 h. Three views were obtained: the superior vena caval transverse, the mid-esophageal four-chamber, and the transgastric mid-papillary short-axis views. Observational data on the feasibility of insertion, complications, image quality, and influence on management were recorded and analyzed.
Results
No failure of probe insertion was observed. The nasogastric tube had to be removed in 17 % of cases. Image quality was judged as adequate or optimal in 91/94 (97 %) of cases in the superior vena caval view, 89/94 (95 %) of cases in the four-chamber view, and 86/94 (91 %) of cases in the short-axis view. The duration of monitoring was 32 ± 23 h, allowing 2.8 ± 1.6 hemodynamic evaluations per patient that led to a mean of 1.4 ± 1.5 therapeutic changes per patient. Among the 263 hemodynamic assessments, 132 (50 %) had a direct therapeutic impact in 62 patients (66 %). Two patients developed lip ulceration from the probe, and two patients had self-limited gastric bleeding.
Conclusion
The single-use miniaturized probe could be inserted in all patients. Image quality was acceptable in the majority of cases, and the information derived from the device was useful in making management decisions in patients with hemodynamic failure on ventilatory support. Further studies are needed to confirm the good tolerance and to compare the new device with other hemodynamic monitoring techniques.
Similar content being viewed by others
Introduction
Transesophageal echocardiography (TEE) has gained popularity and is increasingly used in the intensive care unit (ICU) settings for the hemodynamic assessment of patients with cardiorespiratory compromise [1]. TEE has the unparalleled advantage of providing morphological and functional information on the heart and great vessels in real time in most ventilated critically ill patients, thereby helping the ICU physician to guide acute management [2, 3]. In addition to its valuable diagnostic capability, TEE provides useful information on both the efficacy and tolerance of therapeutic changes decided by the intensivist in light of the bedside interpretation of the examination [4, 5]. Nevertheless, TEE suffers from several limitations, such as the need for training of intensivists who perform and interpret the study, the requirement for a dedicated imaging system, and the discontinuous nature of the hemodynamic assessment requiring repeated probe insertion.
Recent improvements in electronics have allowed the development of miniaturized TEE probes which promise to provide a tool for hemodynamic monitoring in unstable ventilated critically ill patients. These prototype devices have not been widely used in the ICU and their diagnostic ability and potential therapeutic impact, as well as their tolerance, are still unknown. Accordingly, the present pilot study was designed to evaluate the hemodynamic monitoring capability and safety of a commercially available single-use miniaturized TEE probe left in place in ventilated ICU patients assessed for a cardiorespiratory compromise.
Methods
This prospective multicenter study was approved by the Institutional Ethics Committee of Amiens Teaching Hospital that waived the need for informed consent because the four participating centers routinely use TEE for the hemodynamic assessment of ventilated ICU patients with cardiopulmonary compromise as a standard of care.
During a 4-month period, all patients requiring mechanical ventilation for circulatory failure or acute lung injury/acute respiratory distress syndrome (ARDS) [6] and who required TEE for diagnosis and management were eligible for the study. Circulatory failure was defined as the presence of sustained hypotension (systolic blood pressure less than 90 mmHg or a mean arterial pressure less than 65 mmHg) associated with clinical evidence of tissue hypoperfusion which required the use of the vasopressors or inotropes. Patients were excluded if they met at least one of the following criteria: age less than 18 years, pregnancy, contraindication for a TEE study. Blood pressure was continuously monitored invasively in all patients.
The tested single-use miniaturized (diameter 5.5 mm) Federal Drug Agency-approved TEE probe has a European Community mark for 72-h of continuous use. The device produces single-plane two-dimensional imaging and has color Doppler capability (IMACOR, New-York NY, USA). Anteflexion and retroflexion of the tip of the probe are allowed. The probe is connected to a dedicated echographic system which allows the recording of digital loops and performance of basic two-dimensional measurements of areas and distances. The miniaturized TEE probe is inserted blindly, without additional sedation or muscle paralysis, unless necessary, and is left in place without a mouth piece for monitoring purposes at the discretion of the attending ICU physician, according to the patient’s hemodynamic status. The nasogastric tube was checked routinely after probe insertion or removal to rule out any displacement, and feed, if performed, was continued.
All TEE assessments were performed by a highly trained intensivist with expertise in critically care echocardiography. Each hemodynamic assessment purposely relied on three basic transversal TEE views which were systematically screened in each patient for each monitoring step, according to a previously validated protocol [7] based upon the imaging capabilities of the miniaturized TEE probe (Fig. 1). Color Doppler mapping was systematically used to detect regurgitant flow at the level of the mitral, aortic, and tricuspid valves, or to depict the presence of an anatomical intracardiac shunt. No quantitative measurement was performed. The visual interpretation of TEE studies used the recent recommendations for performing critical care echocardiography [8]. Briefly, six parameters were systematically assessed qualitatively in all patients: (1) respiratory variation of the superior vena cava (absent, minor, major) in the superior vena caval transverse view, (2) left ventricular (LV) systolic function (normal, moderately reduced, severely reduced) based on the fractional area change visually assessed in the transgastric short axis view, (3) right ventricular (RV) size (normal, moderately dilated, severely dilated) in the mid-esophageal four-chamber view, (4) presence of a paradoxical septal motion in the transgastric short axis view, (5) presence of a pericardial effusion and of associated cardiac tamponade, and (6) presence of massive valvular regurgitation depicted by color Doppler flow mapping. This standardized hemodynamic assessment allowed the identification of major mechanisms of the cardiopulmonary compromise thereby potentially altering ongoing therapy (Table 1).
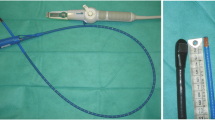
Three basic transverse views used for hemodynamic evaluation and monitoring. Left picture of the single-use miniaturized probe, compared to an usual one. Right a mid-esophageal four-chamber view of the left ventricle. b Transgastric short-axis view of the left ventricle. c Mid-esophageal transverse view of the superior vena cava. LV left ventricle, RV right ventricle, LA left atrium, SVC superior vena cava, RPA right pulmonary artery, Ao aorta
In each patient, the number of attempts to adequately insert the esophageal probe and the need to temporarily withdraw the nasogastric tube were recorded. Image quality obtained for each of the three TEE 0° views was graded as inadequate (precluding any assessment of hemodynamic parameters), adequate (not optimal but sufficient to assess the predefined hemodynamic parameters), or optimal. The duration of TEE probe insertion for hemodynamic monitoring and the number of hemodynamic assessments during the monitoring period were recorded. Therapeutic impact was defined as any therapeutic change directly related to the hemodynamic evaluation during TEE monitoring. Conventional baseline characteristics obtained upon admission were recorded: age, SAPS II score [9], reason for ICU admission, and ICU mortality. Any complication potentially related to the prolonged esophageal intubation was recorded (e.g., bleeding, skin ulceration). The sponsor which provided the single-use TEE probes and dedicated ultrasound systems used in the present study had no access to the data, analysis, and did not participate in the drafting of the manuscript.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation.
Results
Ninety-four patients (60 % men, mean age 64 ± 15 years SAPS II 58 ± 21) were studied. Eighty-one patients (86 %) had circulatory failure (septic shock in 57 patients, cardiogenic shock in 7 patients, post-resuscitation syndrome in 13 patients, hemorrhagic shock in 4 patients), and the remaining 13 patients (14 %) were ventilated for ALI/ARDS. All patients were sedated and fully adapted to the ventilator, and 14 patients (15 %) were paralyzed. ICU mortality was 49 %.
The miniaturized single-used TEE probe was successfully inserted at the first attempt in 78 patients (83 %), and required removal of the nasogastric tube in the remaining 16 patients (17 %) to enhance probe insertion. No additional sedation or paralysis was required. Image quality for the three transverse TEE views is detailed in Fig. 2. Four-chamber and short-axis views were considered as optimal in 68 patients (72 %) and in 60 patients (64 %), respectively. The superior vena caval transverse view was deemed optimal in 61 patients (65 %). All views were concomitantly graded as optimal in 42 patients (45 %), 2/3 views in 20 patients (21 %), and 1/3 view in 19 patients (20 %). A full hemodynamic evaluation could be performed in 85 % of patients. No difference in image quality was observed according to gender or body mass index. Hemodynamic diagnoses based on TEE assessment are reported in Table 1. No patient had massive valvular regurgitation.
Image quality of the three transverse views obtained using the single-use miniaturized TEE probe in 94 ventilated patients with cardiopulmonary compromise. Quality was graded as inadequate (precluding any assessment of hemodynamic parameters), adequate (not optimal but sufficient to assess the predefined hemodynamic parameters), or optimal
The mean period of TEE monitoring using the single-use miniaturized probe was 32 ± 23 h, allowing a mean of 2.8 ± 1.6 hemodynamic evaluations per patient that led to a mean of 1.4 ± 1.5 therapeutic changes per patient. Among the 263 hemodynamic assessments, 132 (50 %) had a direct therapeutic impact in 62 patients (66 %), as follows. Fluid loading was performed in 39 patients (41 %), and inotropic or vasopressor support was initiated or increased in 18 patients (19 %) and 13 patients (14 %), respectively. In eight additional patients (8 %), catecholamines were tapered.
In 17 patients (18 %), the period of monitoring with the single-use miniaturized probe was 6 h or less (Fig. 3), allowing a mean of 1.6 ± 1.0 hemodynamic evaluations per patient that led to a mean of 0.7 ± 0.7 therapeutic changes per patient. In 59 % of patients, the single-use probe had a therapeutic impact. In the remaining 77 patients (82 %), the period of monitoring with the single-use probe was more than 6 h (mean 39 ± 21 h) (Fig. 3). The mean number of hemodynamic evaluations per patient was 3.0 ± 1.6, leading to a direct therapeutic impact in 52 patients (68 %). The mean number of therapeutic changes per patient was 1.6 ± 1.6.
Minor self-limited gastric bleeding without relevant clinical consequence was observed after TEE probe insertion in two patients. In one of these patients, upper endoscopy revealed an esophageal ulceration. In two additional patients, a mechanical ulceration of the superior lip was observed, due to prolonged contact of the miniaturized TEE probe. Technical dysfunction of the single-use TEE probe precluding further imaging occurred in two patients who had already been monitored for 48 h.
Discussion
This multicenter pilot study showed that the tested single-use miniaturized TEE probe was easy to insert, well tolerated when inserted for several days for monitoring purposes, and useful for guiding therapeutic management of ventilated patients with cardiopulmonary compromise. TEE image quality allowed hemodynamic monitoring because 85 % of patients underwent a hemodynamic evaluation based on our previously validated standardized qualitative assessment of central hemodynamics [7]. The mean duration of TEE probe insertion (approximately 32 h) reflects the need of hemodynamic monitoring during the initial course of the cardiopulmonary failure in our critically ill patients. Importantly, hemodynamic assessments resulted in an immediate alteration of ongoing therapy in two-thirds of the patients. Therapeutic impact predominantly consisted in fluid loading, but also helped the attending physician in guiding inotropic or vasopressor support.
The technical limitations of the single-use miniaturized TEE probe accounted for the lower overall imaging quality when compared to that usually obtained with a conventional multiplane TEE probe. This explains why hemodynamic evaluation was not possible to perform in 15 % of patients. In addition, the absence of spectral Doppler capability precluded any quantification of blood flow and cardiac output. Although the miniaturized TTE probe has color Doppler capability, it is not reliable for a precise assessment of the severity of valvular regurgitations but only allows the identification of massive regurgitant jets. These substantial technical limitations, which preclude the performance of a comprehensive hemodynamic assessment, are counterbalanced by the ability to leave the miniaturized TEE probe in place for monitoring purposes over many hours. One postulate of our study was that this new system, despite its ability to measure diameters and areas, has to be used differently for hemodynamic monitoring than a regular probe, with a pure qualitative approach. Whether and how the new system may be combined with conventional multiplane TEE or with other methods of hemodynamic monitoring remains to be studied. A similar qualitative approach to hemodynamic assessment has been previously validated with a larger pediatric monoplane TEE probe used by intensivists without previous knowledge in ultrasound, after limited training [10]. They used a four-chamber and transgastric short-axis view of the heart with the measurement of LV fractional area change. TEE-assessed LV volume status and systolic function led to a fluid challenge or the initiation of cardioactive agent in 40 and in 9 of 100 studied patients, respectively. No therapeutic impact was observed in the remaining 51 % of patients [10]. The lower therapeutic impact reported in that study when compared to the present series is presumably related to the absence of assessment of RV function and cardiac preload-responsiveness, and to the single rather than serial hemodynamic assessment.
We purposely performed qualitative hemodynamic assessments because this approach was best suited for short, repeated evaluations required by a monitoring system and in accordance with the technical characteristics of the tested single-use monoplane TEE probe. In addition, the simple yet robust TEE patterns used in the present study for monitoring purposes have previously been validated in ventilated patients with septic shock against a more comprehensive and quantitative assessment [7]. Because both the diagnostic capacity and therapeutic impact of any imaging modality are heavily influenced by the experience of the operator, data obtained in this pilot study by experienced intensivists with expertise in critical care echocardiography [8] may have overestimated the capacity of the proposed functional, qualitative TEE monitoring of central hemodynamics, if conducted by novice operators.
In the current study, two self-limited gastric bleedings and two mechanical lip ulcerations were observed during the prolonged esophageal insertion of the single-use TEE probe. The relatively sharp tip and rigidity of the miniaturized TEE probe may account for these mild adverse events which did not require specific treatment. Although it has been shown that the prolonged insertion (up to 12 h) of a conventional TEE probe in dogs generated esophageal pressure less than 10 mmHg without gross or microscopic damage to the esophageal wall on pathologic examination [11], TEE is neither used for nor considered a true long-term hemodynamic monitoring system. In contrast, the miniaturized single-use TEE probe tested herein is the size of a nasogastric tube and therefore is adequately suited for prolonged insertion. Importantly, between hemodynamic assessments, the TEE probe should be maintained in a neutral position, because maximal anteflexion of its tip may generate esophageal probe contact pressure of up to 17 mmHg [11].
Our study has several limitations. First, no side-by-side comparison was performed between the examinations performed with the single-use miniaturized TEE probe and a conventional multiplane probe. Even though TEE image quality allowed hemodynamic monitoring in 85 % of patients, all views were optimal in only 45 % of patients and hemodynamic evaluation was unavailable in 15 % of patients. We can anticipate that the greater imaging quality of conventional TEE would have provided additional information which could have influenced the therapeutic management of our patients. Moreover, preload responsiveness was assessed in the transverse view of the great vessels, whereas the visual estimation of respiratory variations of the superior vena cava has been described in the longitudinal 90° view [12]. Second, therapeutic impact of the single-use TEE probe was probably underestimated because it was restricted to therapeutic changes directly related to the information provided by TEE hemodynamic monitoring. However, in most hemodynamic evaluations in which TEE had no direct therapeutic impact, this monitoring allowed the confirmation that ongoing treatment was well adapted to the hemodynamic profile. Third, we cannot extend our results to other ICU practices because the current investigators all have an extended experience in conducting TEE studies [2, 4, 5]. Accordingly, the use of the tested miniaturized single-use TEE probe in less experienced hands may not provide similar information, and may therefore have a lesser therapeutic impact. The learning curve required for a novice operator to efficiently conduct a hemodynamic monitoring using the tested device remains to be determined. Fourth, gastric fibroscopy was not systematically performed in our patients and the probe was left in place for an average of 32 h only. Consequently, the reported adverse events potentially related to the prolonged use of the miniaturized TEE probe may have been underestimated. Nevertheless, no side effect requiring a specific treatment was reported in this series. Fifth, the nasal insertion has not been tested in the current study, as was previously done with another miniaturized monoplane, multiple-use, TEE probe [13]. Finally, interobserver reproducibility was not tested, but all investigators were experts in TEE with extensive experience in this area.
In conclusion, in expert hands, the tested single-use miniaturized TEE probe provided relevant information for the hemodynamic monitoring of ventilated ICU patients with cardiorespiratory compromise and had a therapeutic impact in the majority of patients. Further studies are needed to confirm the tolerance of long-term insertion of this miniaturized TEE probe, to determine the learning curve required for its proper use by ICU physicians who are novices in ultrasound, and to compare the new device with other hemodynamic monitoring techniques before making any recommendations. Future technical improvement of the tested device promises to further increase the clinical value of this novel ultrasound-based functional approach to hemodynamic monitoring.
References
Vieillard-Baron A, Slama M, Cholley B, Janvier G, Vignon P (2008) Echocardiography in the intensive care unit: from evolution to revolution? Intensive Care Med 34:243–249
Vieillard-Baron A, Prin S, Chergui K, Dubourg O, Jardin F (2003) Hemodynamic instability in sepsis: bedside assessment by Doppler echocardiography. Am J Respir Crit Care Med 168:1270–1276
Etchecopar-Chevreuil C, François B, Clavel M, Pichon N, Gastinne H, Vignon P (2008) Cardiac morphological and functional changes during early septic shock: a transesophageal echocardiographic study. Intensive Care Med 34:250–256
Vignon P, Mentec H, Terré S, Gastinne H, Guéret P, Lemaire F (1994) Diagnostic accuracy and therapeutic impact of transthoracic and transesophageal echocardiography in mechanically ventilated patients in the ICU. Chest 106:1829–1834
Slama M, Novara A, Van de Putte P, Diebold B, Safavian A, Safar M, Ossart M, Fagon JY (1996) Diagnostic and therapeutic implications of transesophageal echocardiography in medical ICU patients with unexplained shock, hypoxemia, or suspected endocarditis. Intensive Care Med 22:916–922
Bernard G, Artigas A, Brigham M, Carlet J, Falke K, Hudson L, Lamy M, Le Gall JR, Morris A, Spragg R (1994) The American-European consensus conference on ARDS. Definition, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 149:818–824
Vieillard-Baron A, Charron C, Chergui K, Peyrouset O, Jardin F (2006) Bedside echocardiographic evaluation of hemodynamics in sepsis: is a qualitative evaluation sufficient? Intensive Care Med 32:1547–1552
Mayo PH, Beaulieu Y, Doelken P, Feller-Kopman D, Harrod C, Kaplan A, Oropello J, Vieillard-Baron A, Axler O, Lichtenstein D, Maury E, Slama M, Vignon P (2009) American College of Chest Physicians/La Societe de Reanimation de Langue Française. Statement on competence in critical care ultrasonography. Chest 135:1050–1060
Le Gall JR, Lemeshow S, Saulnier F (1993) A new simplified acute physiology score (SAPS II) based on a European/North American multicenter study. JAMA 270:2957–2963
Benjamin E, Griffin K, Leibowitz A, Manasia A, Oropello J, Geffroy V, DelGiudice R, Hufanda J, Rosen S, Goldman M (1998) Goal-directed transesophageal echocardiography performed by intensivists to assess left ventricular function: comparison with pulmonary artery catheterization. J Cardiothorac Vasc Anesth 12:10–15
Urbanowicz JH, Kernoff RS, Oppenheim G, Parnagian E, Billingham ME, Popp RL (1990) Transesophageal echocardiography and its potential for esophageal damage. Anesthesiology 72:40–43
Vieillard-Baron A, Chergui K, Rabiller A, Peyrouset O, Page B, Beauchet A, Jardin F (2004) Superior vena caval collapsibility as a gauge of volume status in ventilated septic patients. Intensive Care Med 30:1734–1739
Spencer KT, Krauss D, Thurn J, Mor-Avi V, Poppas A, Vignon P, Connor BG, Lang RM (1997) Transnasal transesophageal echocardiography. J Am Soc Echocardiogr 10:728–737
Acknowledgments
We gratefully thank IMACOR for providing the single-use.
Conflicts of interest
All the authors had no financial conflicts of interest to declare related to the study.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Investigators: Pauline Champy, MD, Marc Clavel, MD, Bruno François, MD, Nicolas Pichon, MD, Medical-surgical Intensive Care Unit, CHU de Limoges, 2 avenue Martin Luther King, 87042 Limoges, France; Center of Clinical Investigation, INSERM 0801, 87042 Limoges, France. Laurent Bodson, MD, Siu-Ming Au, MD, Intensive Care Unit, Section Thorax–Vascular Diseases–Abdomen–Metabolism, University Hospital Ambroise Paré, 9 avenue Charles de Gaulle, 92104 Boulogne, France.
Rights and permissions
About this article
Cite this article
Vieillard-Baron, A., Slama, M., Mayo, P. et al. A pilot study on safety and clinical utility of a single-use 72-hour indwelling transesophageal echocardiography probe. Intensive Care Med 39, 629–635 (2013). https://doi.org/10.1007/s00134-012-2797-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-012-2797-4