Abstract
Aims/hypothesis
The use of HbA1c for the diagnosis of diabetes is now widely advocated despite caveats to its use. Anaemia is cited as a major confounder to this use; however, the effect of erythrocyte indices and to what degree anaemia influences HbA1c levels is not known.
Methods
A systematic electronic database search of MEDLINE, EMBASE, the Cumulative Index to Nursing & Allied Health Literature (CINAHL) and the Cochrane Library was conducted for relevant articles published between January 1990 and May 2014. Included studies had at least one measurement of HbA1c and glucose, and a least one index of haematinic deficiency, involving non-pregnant adults, not known to have diabetes.
Results
A total of 12 articles from 544 were included. The majority of studies focused on iron deficiency anaemia (IDA) and, in general, demonstrated that the presence of iron deficiency with or without anaemia led to an increase in HbA1c values compared with controls, with no concomitant rise in glucose indices. Data on the effects of other indices of erythrocyte abnormalities on HbA1c are limited but show a possible decrease in HbA1c values with non-iron deficiency forms of anaemia.
Conclusions/interpretation
HbA1c is likely to be affected by iron deficiency and IDA with a spurious increase in HbA1c values; conversely, non-IDA may lead to a decreased HbA1c value. This may lead to confusion when diagnosing diabetes using HbA1c. This review clearly identifies the need for more evidence, especially in identifying the types and degrees of anaemia likely to have significant impact on the reliability of HbA1c.
Similar content being viewed by others
Introduction
The traditional role of HbA1c analysis has been for assessing glycaemic control in patients with diabetes. The results of seminal studies [1, 2] demonstrated that early, intensive glycaemic control could significantly reduce the risk of a range of diabetes-related complications, and permitted the establishment of precise HbA1c target values for treatment goals [3].
More recently, there has been a move towards the use of HbA1c for the diagnosis of type 2 diabetes. The WHO and the ADA have both advocated the use of HbA1c for diagnosing type 2 diabetes, at a value of 6.5% (48 mmol/mol) [4, 5].
Further to the recommendations of the WHO, the UK issued an expert position statement on the application of these recommendations in clinical practice in the UK [6]. One key factor thought to be a confounder in the use of HbA1c is an altered erythrocyte lifespan, in particular due to anaemia. The WHO defines anaemia in adults as 120 g/l Hb in non-pregnant women and 130 g/l in men [7]. It is widely purported that haemolytic anaemia can lead to decreased HbA1c values due to reduced erythrocyte lifespan, and iron deficiency anaemia (IDA) may result in increased HbA1c values due to an elongation of the erythrocyte lifespan. However, it is not known to what degree alterations in erythrocyte indices affect HbA1c values especially around the diagnostic cut point of 6.5% (48 mmol/mol) or the degree of abnormality severity required to result in a significant change. With approximately 29% of non-pregnant women worldwide having anaemia in 2011 [8], this translates to a significant number of people where the use of HbA1c for diagnosis of diabetes may be precluded.
Since the publication of the recommendations, there has been a demand for clarity on this topic. The key questions asked are: ‘At what level of anaemia should I not use HbA1c for diagnosis?’ and ‘Should I routinely screen patients for anaemia when using HbA1c for diagnosis and if so, what test should I use?’
This systematic review aims to address the above questions by assessing the available evidence on the impact of abnormalities of erythrocyte indices and anaemia, on HbA1c levels around the diagnostic cut off point of 6.5% (48 mmol/mol).
Methods
This systematic review is registered on Prospero (registration no. CRD42013005251). Approval of an ethics committee was not required.
Study identification
An electronic database search for relevant articles published between January 1990 and May 2014 was conducted using the following databases: MEDLINE, EMBASE, Cumulative Index to Nursing & Allied Health Literature (CINAHL) and The Cochrane Library.
The search was carried out using a combination of keywords and MESH terms or EMtree terms depending on the particular database (see Electronic Supplementary Material [ESM] Methods for details).
In general, the search strategy consisted of: (1) HbA1c/glycated haemoglobin; (2) iron deficiency; (3) folate, B12 deficiency; and (4) anaemia.
Study selection
Results from all searches were combined and duplicates were removed. Two investigators (EE and GS) evaluated the title and abstract of each reference identified by the search. Inclusion criteria are fully detailed in Table 1; for inclusion, all studies required at least one HbA1c value with fasting plasma glucose (FPG) or OGTT values and erythrocyte and/or iron indices measured. This was to ensure that any changes in HbA1c value were due to changes in the erythrocytes and not due to changes in glycaemia. Any that were clearly irrelevant were removed and the full texts of remaining articles were retrieved. Upon further scrutiny of the full texts, those that did not meet the inclusion criteria were subsequently excluded. The reference lists of included articles were reviewed in order to identify any further articles of relevance to the subject area, and that met the inclusion criteria.
Data extraction
Two investigators extracted the results from each article using a data extraction form based on the pre-defined study inclusion criteria (Table 1). Any disagreement regarding study selection was resolved by means of consensus, involving a third investigator, according to a-priori agreed criteria. The main categories extracted included: author, year, title, study overview, patient characteristics, definition of diabetes, definition of anaemia and key conclusions, as detailed in Table 2 and ESM Table 2.
Quality assessment of included articles
The methodological quality of each study was independently rated by two investigators (EE and GS—who were not blinded to author, journal or institution) according to the Scottish Intercollegiate Guidelines Network (SIGN) criteria [9]. Quality assessment was not used as an exclusion criterion, though articles conducted to high standards in minimising the risk of confounding were noted (ESM Table 1).
Data analysis
Variation within study designs, and heterogeneity of results, meant that the data was unsuitable for meta-analysis. Instead, the existing analyses described in the included articles were extracted and reported in a systematic format, as a narrative synthesis of the main outcomes and results of each study. In line with current recommendations [10, 11], the HbA1c units in the main text have been converted to SI units using the master equation.
Results
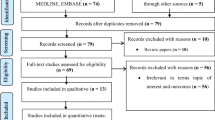
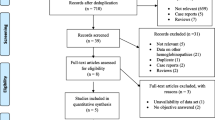
The electronic database searches identified 451 potentially relevant, unique articles. Titles/abstracts of the 451 articles were reviewed and 408 were excluded based on evaluation of the title and/or abstract. The remaining 43 full text articles were reviewed, 31 were excluded upon further reading. The residual 12 were approved and quality assessed using the SIGN criteria. The main reasons for exclusion were irrelevant or incomplete data when compared with the inclusion criteria. No additional articles were identified through review of the reference lists (see Fig. 1 for flow chart).
Narrative analysis of included studies
Cross-sectional studies in patients with and without diabetes
Kim et al [12] investigated the influence of iron deficiency on HbA1c distribution among adults who were not known to have diabetes, over 7 years of the National Health and Nutrition Examination Survey (NHANES). Of the 6,666 female participants, 13.7% had iron deficiency and 30% of these had IDA. A much lower proportion of males (1.6%) had iron deficiency and 33% of these had IDA. When HbA1c values in women were adjusted for age and ethnicity, the difference between iron deficient and non-iron deficient became significant (5.33% vs 5.27% [35 mmol/mol vs 34 mmol/mol], p = 0.002). The authors found that iron deficiency in women of reproductive age was associated with a shift in HbA1c from <5.5% to 5.5–6.0% (<37 mmol/mol to 37–42 mmol/mol), although no association was found at higher levels, possibly owing to the lower number of participants in those groups. After adjusting for age and ethnicity, the authors concluded that HbA1c was higher in iron-deficient individuals and was likely to result in an upward shift of HbA1c distributions.
Ford et al [13] evaluated 1999–2002 NHANES data sets but included analysis of both IDA and non-IDA in participants both with and without diabetes. They found that non-diabetic participants with low Hb but normal iron levels had lower HbA1c values (5.16% [33 mmol/mol]) than those with normal Hb and normal iron levels (5.31% [35 mmol/mol], p < 0.001). In contrast, those with normal Hb but low iron values had borderline higher HbA1c values than normal participants (5.39% [35 mmol/mol], p = 0.061). In addition, in all participants without diabetes, the HbA1c values increased with increasing Hb levels ranging from a mean HbA1c of 5.18% (33 mmol/mol) at Hb <100 g/l through to 5.50% (37 mmol/mol) with Hb >170 g/l. The authors advocate caution when interpreting HbA1c values near diagnostic cut points when Hb levels are high or low. The study was limited by low numbers of iron deficiency and/or anaemia cases and few severe cases but the authors suggest that the likely impact of haemoglobin concentration on HbA1c values was an approximate change of HbA1c 0.2% (2.2 mmol/mol), between the extremes of Hb levels.
Studies comparing prevalence of diabetes and prediabetes diagnosed by glucose or HbA1c
Two studies [14, 15] compared the prevalence of diabetes/prediabetes determined by glucose-based criteria and HbA1c-based criteria. Hardikar et al [14] compared HbA1c and OGTT determined diabetes rates in a cohort of young adults in India. The authors found that the prevalence of diabetes or prediabetes was higher with HbA1c criteria than by standard OGTT (25.9% vs 10.4%). Within a subgroup of anaemic (IDA, B12 and folate deficiency) patients, the discordance was even greater with 33% classed as prediabetes or diabetes by HbA1c compared with 12% by OGTT.
Son et al [15] grouped 329 Korean participants, not previously known to have diabetes, by diabetes status according to OGTT results. Participants were grouped as normal, prediabetes or diabetes, HbA1c levels were then compared in each group for both anaemic and non-anaemic participants. In the normoglycaemic group, HbA1c values were found to be the same in both anaemic and non-anaemic groups. In the prediabetes group, HbA1c values were found to be higher in the anaemic group compared with controls (p = 0.05) and borderline significant in the diabetes group. The authors acknowledge that small sample sizes and a lack of definition of types of anaemia are confounding factors in their study.
Studies comparing HbA1c and glucose values in patients without diabetes, with and without IDA
One study [16] compared the HbA1c values in 50 individuals with IDA, not previously known to have diabetes, with non-anaemic healthy controls. This was a short article with limited description or discussion but essentially both FPG and postprandial glucose values were not significantly different between the IDA and control groups. HbA1c values however were significantly higher in the IDA group (mean 7.6 ± 0.5% [60 ± 5.5 mmol/mol]) compared with the control group (5.5 ± 0.8% [37 ± 8.7 mmol/mol]; p < 0.001).
Studies evaluating the effects of treatment, to resolve anaemia, on HbA1c
Four studies [17–20] evaluated HbA1c levels in non-diabetic patients, pre and post treatment for anaemia. Each study included patients treated with oral iron replacement therapy, three studies [17–19] reported a significant fall in HbA1c values after treatment with iron replacement over periods of 9–20 weeks.
Coban et al [17] saw a fall from an HbA1c of 7.2 ± 0.8% (55 ± 8.7 mmol/mol) pre treatment to 6.2 ± 0.6% (44 ± 6.6 mmol/mol) post 3 months treatment but this was still not as low as the non-anaemic control group 5.2 ± 0.2% (33 ± 2.2 mmol/mol); however, the iron indices had not fully normalised compared with controls at that point in time either. The same pattern was demonstrated by El-Agouza et al [18] where patients were followed for 20 weeks and HbA1c fell steadily over that period from 6.15 ± 0.62% to 5.25 ± 0.45% (44 ± 6.8 mmol/mol to 34 ± 4.9 mmol/mol). However, there were no control participants included for comparison and it would be of interest to know the point at which HbA1c values plateaued post treatment. Similarly, a small study (n = 10) [19], showed a significant decline (p < 0.01) in HbA1c values by 3 weeks of treatment and the values remained below baseline values after 9 weeks, despite an upward rebound.
Interestingly, a study by Sinha et al [20] showed the converse pattern of results in 50 patients with moderate to severe IDA (mean ± SD Hb, 62 ± 1 g/l). The mean HbA1c was significantly lower in the anaemic group compared with the non-anaemic controls (4.6 ± 0.6% vs 5.5 ± 0.6%; 27 ± 6.6 mmol/mol vs 37 ± 6.6 mmol/mol). After 2 months of treatment for IDA, the HbA1c values were significantly higher than the controls raising the question of whether severe iron deficiency has the same effect on glycation as more mild anaemia.
Studies investigating the effect of menstruation on HbA1c levels
Three studies [21–23] investigated the impact of the premenopausal state on HbA1c values. Behan [21] compared Hb values in non-diabetic, non-anaemic premenopausal women with postmenopausal white women. The study found that Hb levels were significantly lower in premenopausal women, despite no reported history of anaemia. HbA1c values were not significantly different between the two groups but the correlation between HbA1c and FPG was weaker in the premenopausal group. The author suggests that the varying effects of menstruation on iron metabolism could lead to less reliable HbA1c and FPG correlations.
Koga [22, 23] and colleagues also looked at the effect of the menopause on HbA1c values. The initial study identified a significant difference in HbA1c levels between pre- and postmenopausal women (4.9 ± 0.2% vs 5.1 ± 0.3% [30 ± 2.2 mmol/mol vs 32 ± 2.2 mmol/mol] p < 0.0001). In premenopausal women, mean corpuscular haemoglobin (MCH) and mean corpuscular volume (MCV) were inversely correlated with HbA1c but this association was not significant in postmenopausal women. The authors suggest that every 1 pg decrease in MCH correlated with a 0.03% (0.3 mmol/mol) increase in HbA1c value and that erythrocyte indices influence HbA1c values in premenopausal women in the absence of overt anaemia. Koga et al [23] also looked at the effect of iron deficiency and IDA compared with iron sufficiency on HbA1c and glycated albumin values in premenopausal women. Iron deficiency and IDA were both associated with a significant increase in HbA1c levels with iron metabolism indices being significantly negatively associated with HbA1c. This association was not present in glycated albumin levels across the three iron status groups, essentially indicating that iron metabolism rather than glycaemic changes influence changes in HbA1c in premenopausal women.
Studies evaluating erythrocyte indices and markers of iron status
All studies were reviewed for data on erythrocyte indices and markers of iron status as these provide further insight into which adjunct tests may support the use of HbA1c for diagnosis. Data extracted are detailed in Table 3. Combinations of the following indices were measured: Hb, packed cell volume (PCV), MCV, MCH, MCH concentration (MCHC), ferritin, transferrin saturation (TSAT), reticulocytes, red cell distribution width (RDW) and erythrocyte protoporphyrin. Some studies only used the values to identify or exclude patients with iron deficiency or anaemia whereas others correlated changes in erythrocyte indices with changes in HbA1c value. Of the studies that focused on IDA, three studies compared values pre and post treatment with iron replacement and two compared values in patients with anaemia against control participants. All studies demonstrated an increase in MCV and MCH with treatment or higher levels in controls compared with anaemia patients. Two studies [14, 22] demonstrated an inverse correlation between HbA1c and Hb, MCV and MCH levels. Overall, the data indicate that iron deficiency, demonstrated by low Hb, low MCV and low MCH, is associated with increased HbA1c levels both with and without overt anaemia. In addition, normal MCV and MCH with low Hb would not be associated with increased HbA1c levels but rather a decrease in values.
Ferritin was measured in nine studies [12–14, 16–18, 20, 21, 23]. Of these, most showed an increase in ferritin levels post treatment for anaemia and also showed that ferritin levels were lower in iron-deficient participants compared with controls. Three studies [14, 16, 23] showed that ferritin was a negative predictor of HbA1c and one study [13] showed a small but significant positive correlation between HbA1c and ferritin. Generally, ferritin was utilised as a marker of iron deficiency rather than analysed as an independent predictor of HbA1c values; where the latter was done, the results were mixed.
Discussion
Does anaemia affect HbA1c?
Generally, the studies investigating the effect of anaemia and abnormalities of erythrocyte indices on HbA1c analysis are limited to small sample groups and two studies that both analysed the same large cross-sectional population study. It is apparent that IDA can have a significant impact on HbA1c values with most studies suggesting a spuriously high HbA1c in IDA compared with other markers of glycaemia. The exception to this is the study by Sinha et al [20], which found low values of HbA1c in severe cases of IDA that increased with iron replacement therapy. The authors did not discuss why their data conflicts with other studies but it may be due to the severity of anaemia as the participants in this study had low mean Hb levels (62 g/l); the duration of anaemia was not given. Together, these data support the hypothesis that iron deficiency per se may cause elevated HbA1c values, irrespective of anaemia.
Limited data indicate that non-IDAs also affect HbA1c values to a varying degree [13, 14, 19]. Ford et al [13] showed that in patients without diabetes, with low Hb but normal iron levels had significantly lower HbA1c values than those with normal iron and normal Hb (see Table 2). The difference between the non-IDA and control values was greater than was observed between IDA and iron deficiency values and controls, suggesting that HbA1c may be spuriously elevated in iron deficiency and spuriously depressed in non-IDAs. Ideally, any further studies would separate out iron deficiency and non-iron deficiency cases prior to analysis as there is the potential to null the data by combing the two pathologies in one evaluation.
Hardikar et al [14] investigated a population where of those with anaemia 30.8% had vitamin B12 deficiency, 15% had folate deficiency and 30% had multiple nutrient deficiencies. In multivariate analysis, B12 and folate were not significantly related to HbA1c levels but this may be due to the small sample size analysed. Where mixed nutrient deficiencies are observed, the combination of pathologies will lead to variable and unpredictable effects on HbA1c levels depending on the relative degree of each nutrient deficiency.
To what degree do abnormalities of erythrocyte indices affect HbA1c values?
The two evaluations of the cross-sectional NHANES data showed that having iron deficiency or IDA increased the odds of having an HbA1c value shift upwards from <5.5% (<37 mmol/mol) to 5.5–5.9% (37–41 mmol/mol) [12, 13]. Hardikar et al [14] demonstrated that markers of microchromic anaemia were associated with increased HbA1c values and also more patients diagnosed with prediabetes or diabetes by HbA1c compared with glucose indices. In patients with non-IDA, there was a marked shift downwards in apparent distribution of HbA1c results [13]. Son et al [15] demonstrated that IDA in the patients with prediabetes defined by glucose levels had a mean HbA1c of 6.4% vs 6.1% (46 mmol/mol vs 43 mmol/mol) in controls; this is sufficient to re-categorise some patients from prediabetes to diabetes in the anaemic group.
Other studies which looked more specifically at HbA1c levels in patients with or without anaemia showed differences of up to 2.1% (23 mmol/mol) HbA1c increase with IDA [16] and studies measuring HbA1c pre and post treatment for anaemia showed changes as large as −1.2% (−13 mmol/mol) HbA1c post treatment [17].
What does this mean on a wider scale?
Recent data has shown a surge in the incidence and prevalence of young onset obesity, many of whom are premenopausal females, at increased risk of iron deficiency. There has been a concomitant increase in HbA1c values in these participants considered at high risk of diabetes, owing to factors such as obesity [24]. Data from England shows that the prevalence of prediabetes rose from 11.6% in 2003 to 35.3% in 2011 [25] and in view of the rising prevalence of obesity, we would anticipate to identify a larger proportion of patients, with higher HbA1c values. If the estimated upward shift in HbA1c values seen with iron deficiency is combined with the apparent increased prevalence of prediabetes, it may result in a significant number of patients where the combination of the two will be sufficient to shift HbA1c values to move from a diagnosis of prediabetes to diabetes.
Clinical use and relevance
One of the main questions still to answer is how to apply this information to everyday clinical practice. In addition to published guidance [4–6], we suggest the following:
-
1.
During monitoring of people with diabetes, when glucose and HbA1c are discordant, consider abnormalities of erythrocyte indices.
-
2.
When HbA1c is normal/elevated but Hb is low, do not assume that HbA1c is falsely elevated—check erythrocyte indices, in particular MCV and MCH; if low, consider iron deficiency by TSAT or ferritin. If MCV and MCH are not low then consider other forms of anaemia—HbA1c may be falsely decreased in these cases.
-
3.
Iron deficiency, as well as IDA, may be sufficient to cause a change in HbA1c values; this is highly relevant in women of childbearing age.
-
4.
If abnormalities of erythrocyte indices or anaemia are identified, consider correction of the abnormality before using HbA1c for diagnosis or monitoring. The studies included in this review suggest that it may take up to 6 months after treatment is initiated to normalise erythrocyte indices. RDW will provide an additional indicator of normalisation of the erythrocyte population and erythrocyte lifespan.
Ferritin as a marker of iron status is useful if it is low but would not rule out iron deficiency if it was found to be normal or elevated as ferritin is an acute phase reactant and inflammation may mask a true low ferritin. Raj and Rajan [26] investigated 86 patients with type 2 diabetes mellitus and demonstrated that serum ferritin positively correlated with HbA1c and was increased with increasing duration of disease, indicating that poor glycaemic control can contribute to elevated ferritin levels independent of iron status. In situations of increased inflammation, alternative measures of iron status such as TSAT or total iron binding capacity should be considered.
Changes in erythrocyte lifespan even with normal haematological indices can affect HbA1c values [27], therefore, it is important to show that the erythrocyte population is stable after treatment for abnormalities before using HbA1c. Erythrocyte indices including RDW may assist with this.
Further research questions
Although it is clear that anaemia may influence HbA1c results, further studies to identify the roles of erythrocyte indices as appropriate adjunct analyses to identify patients where this is an issue, are needed. Future studies should consider the effect of erythrocyte indices rather than anaemia alone. In particular, studies should aim to include sufficient participants to differentiate between the effects of the type and severity of erythrocyte abnormalities/anaemia on HbA1c values. This may be achieved through a combination of population based studies and intervention studies where changes in HbA1c values are assessed in relation to therapy for erythrocyte abnormalities and anaemias.
Strengths and limitations of this study
This review is limited to a selection of small study groups and two separate analyses of the NHANES survey data from overlapping time periods. The conclusions drawn are limited by a lack of robust evidence from a significant number of the included studies. The quality analysis of the included studies has highlighted shortfalls and confounders in most of the cases, which would need to be addressed in future studies—in particular, a lack of power to confer significance on many of the findings.
Notes on standardisation of HbA1c methods
International standardisation of HbA1c was achieved in 2002 [28–30]. An International HbA1c Consensus Committee was formed which agreed that HbA1c values should be reported in both SI units (mmol/mol) and converted to % units via the master equations established by the International Federation for Clinical Chemistry and Laboratory Medicine (IFCC) and designated comparison methods [10, 11]. All studies reported in this review either stated that they were aligned to the NGSP or did not state any performance information for the HbA1c analysis. In light of this, even if there was sufficient data for meta-analysis, it is likely that a lack of standardisation of results would be a major confounder. Any further studies should endeavour to ensure that all HbA1c measurements are performed in alignment with the IFCC and clear quality data should be provided in the reports.
Conclusion
It is clear from the limited number of studies, many with low participant numbers, that the subject of anaemia and HbA1c warrants further investigation. Generally, the studies described demonstrate that abnormalities of erythrocyte indices are a considerable confounder in the analysis of HbA1c and there is currently insufficient data to fully inform clinicians and scientists on how to address this in clinical practice. However, we have made some suggestions to facilitate its use whilst clarity is awaited.
The key questions that are still to be answered are whether anaemia and erythrocyte abnormalities will have a significant impact on the diagnosis of diabetes using HbA1c in the general population—something that is now widely performed.
Abbreviations
- CINAHL:
-
Cumulative Index to Nursing & Allied Health Literature
- FPG:
-
Fasting plasma glucose
- IDA:
-
Iron deficiency anaemia
- IFCC:
-
International Federation for Clinical Chemistry and Laboratory Medicine
- MCH:
-
Mean corpuscular haemoglobin
- MCHC:
-
Mean corpuscular haemoglobin concentration
- MCV:
-
Mean corpuscular volume
- NHANES:
-
National Health and Nutrition Examination Survey
- PCV:
-
Packed cell volume
- RDW:
-
Red cell distribution width
- SIGN:
-
Scottish Intercollegiate Guidelines Network
- TSAT:
-
Transferrin saturation
References
The Diabetes Control and Complications Trial Research Group (1993) The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 329:977–986
UK Prospective Diabetes Study (UKPDS) Group (1998) Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 352:837–853
Inzucchi SE, Bergenstal RM, Buse JB et al (2012) Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 35:1364–1379
World Health Organization (2011) Use of glycated haemoglobin (HbA1c) in the diagnosis of diabetes mellitus (abbreviated report of a WHO consultation)
American Diabetes Association (2013) Standards of medical care in diabetes–2013. Diabetes Care 36(Suppl 1):S11–S66
John WG (2012) Use of HbA1c in the diagnosis of diabetes mellitus in the UK. The implementation of World Health Organization guidance 2011. Diabet Med 29:1350–1357
United Nations Children’s Fund, United Nations University, World Health Organization (2001) Iron deficiency anaemia assessment, prevention, and control: a guide for programme managers. Available from www.who.int/nutrition/publications/en/ida_assessment_prevention_control.pdf
Stevens GA, Finucane MM, De-Regil LM et al (2013) Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995-2011: a systematic analysis of population-representative data. Lancet Glob Health 1:e16–25
Lowe G, Twaddle S (2005) The Scottish Intercollegiate Guidelines Network (SIGN): an update. Scot Med J 50:51–52
Consensus Committee (2007) Consensus statement on the worldwide standardization of the hemoglobin A1C measurement: the American Diabetes Association, European Association for the Study of Diabetes, International Federation of Clinical Chemistry and Laboratory Medicine, and the International Diabetes Federation. Diabetes Care 30:2399–2400
Hanas R, John G, International Hb ACC (2010) 2010 consensus statement on the worldwide standardization of the hemoglobin A1c measurement. Clin Chem 56:1362–1364
Kim C, Bullard KM, Herman WH, Beckles GL (2010) Association between iron deficiency and A1C Levels among adults without diabetes in the National Health and Nutrition Examination Survey, 1999–2006. Diabetes Care 33:780–785
Ford ES, Cowie CC, Li C, Handelsman Y, Bloomgarden ZT (2011) Iron-deficiency anemia, non-iron-deficiency anemia and HbA1c among adults in the US. J Diabetes 3:67–73
Hardikar PS, Joshi SM, Bhat DS et al (2012) Spuriously high prevalence of prediabetes diagnosed by HbA(1c) in young Indians partly explained by hematological factors and iron deficiency anemia. Diabetes Care 35:797–802
Son JI, Rhee SY, Woo JT et al (2013) Hemoglobin A1c may be an inadequate diagnostic tool for diabetes mellitus in anemic subjects. Diabetes Metab J 37:343–348
Shanthi B, Revathy C, Manjula Devi AJ, Subhashree (2013) Effect of iron deficiency on glycation of haemoglobin in nondiabetics. J Clin Diagn Res 7:15–17
Coban E, Ozdogan M, Timuragaoglu A (2004) Effect of iron deficiency anemia on the levels of hemoglobin A1c in nondiabetic patients. Acta Haematol 112:126–128
El-Agouza I, Abu Shahla A, Sirdah M (2002) The effect of iron deficiency anaemia on the levels of haemoglobin subtypes: possible consequences for clinical diagnosis. Clin Lab Haematol 24:285–289
Gram-Hansen P, Eriksen J, Mourits-Andersen T, Olesen L (1990) Glycosylated haemoglobin (HbA1c) in iron- and vitamin B12 deficiency. J Intern Med 227:133–136
Sinha N, Mishra TK, Singh T, Gupta N (2012) Effect of iron deficiency anemia on hemoglobin A1c levels. Ann Lab Med 32:17–22
Behan KJ (2006) Cessation of menstruation improves the correlation of FPG to hemoglobin A1c in Caucasian women. Clin Lab Sci 19:225–230
Koga M, Morita S, Saito H, Mukai M, Kasayama S (2007) Association of erythrocyte indices with glycated haemoglobin in pre-menopausal women. Diabet Med 24:843–847
Koga M, Saito H, Mukai M, Matsumoto S, Kasayama S (2010) Influence of iron metabolism indices on glycated haemoglobin but not glycated albumin levels in premenopausal women. Acta Diabetol 47(Suppl 1):S65–S69
Selvin E, Parrinello CM, Sacks DB, Coresh J (2014) Trends in prevalence and control of diabetes in the United States, 1988–1994 and 1999–2010. Ann Intern Med 160:517–525
Mainous AG 3rd, Tanner RJ, Baker R, Zayas CE, Harle CA (2014) Prevalence of prediabetes in England from 2003 to 2011: population-based, cross-sectional study. BMJ Open 4:e005002
Raj S, Rajan GV (2013) Correlation between elevated serum ferritin and HbA1c in type 2 diabetes mellitus. Int J Res Med Sci 1:12–15
Cohen RM, Franco RS, Khera PK et al (2008) Red cell life span heterogeneity in hematologically normal people is sufficient to alter HbA1c. Blood 112:4284–4291
Hoelzel W, Weykamp C, Jeppsson JO et al (2004) IFCC reference system for measurement of hemoglobin A1c in human blood and the national standardization schemes in the United States, Japan, and Sweden: a method-comparison study. Clin Chem 50:166–174
Jeppsson JO, Kobold U, Barr J et al (2002) Approved IFCC reference method for the measurement of HbA(1c) in human blood. Clin Chem Lab Med 40:78–89
Weykamp C, John WG, Mosca A et al (2008) The IFCC Reference Measurement System for HbA1c: a 6-year progress report. Clin Chem 54:240–248
Acknowledgements
We would like to thank R. Curtis (Library Services, University of Nottingham, Nottingham, UK) for her help and guidance in constructing the database searches.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Duality of interest statement
The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement
All of the authors listed have made a substantial contribution to the production of this article. EE, II, KD, EK and WGJ all contributed to the concept of the work; EE, GS and II designed the data collection; EE and GS collected and analysed the data. All authors revised the manuscript critically for important intellectual content and approved the final version to be submitted/published. EE drafted the manuscript. EE is the guarantor of this work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM Methods
(PDF 6 kb)
ESM Table 1
(PDF 310 kb)
ESM Table 2
(PDF 248 kb)
Rights and permissions
About this article
Cite this article
English, E., Idris, I., Smith, G. et al. The effect of anaemia and abnormalities of erythrocyte indices on HbA1c analysis: a systematic review. Diabetologia 58, 1409–1421 (2015). https://doi.org/10.1007/s00125-015-3599-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-015-3599-3