Abstract
The consensus algorithm for the medical management of type 2 diabetes was published in August 2006 with the expectation that it would be updated, based on the availability of new interventions and new evidence to establish their clinical role. The authors continue to endorse the principles used to develop the algorithm and its major features. We are sensitive to the risks of changing the algorithm cavalierly or too frequently, without compelling new information. An update to the consensus algorithm published in January 2008 specifically addressed safety issues surrounding the thiazolidinediones. In this revision, we focus on the new classes of medications that now have more clinical data and experience.
Similar content being viewed by others
Introduction
The epidemic of type 2 diabetes mellitus and the recognition that achieving specific glycaemic goals can substantially reduce morbidity have made the effective treatment of hyperglycaemia a top priority [1–3]. While the management of hyperglycaemia, the hallmark metabolic abnormality associated with type 2 diabetes, has historically taken centre stage in the treatment of diabetes, therapies directed at other coincident features, such as dyslipidaemia, hypertension, hypercoagulability, obesity and insulin resistance, have also been a major focus of research and therapy. Maintaining glycaemic levels as close to the non-diabetic range as possible has been demonstrated to have a powerful beneficial effect on diabetes-specific microvascular complications, including retinopathy, nephropathy and neuropathy, in the setting of type 1 diabetes [4, 5]; in type 2 diabetes, more intensive treatment strategies have likewise been demonstrated to reduce microvascular complications [6–8]. Intensive glycaemic management resulting in lower HbA1c levels has also been shown to have a beneficial effect on cardiovascular disease (CVD) complications in type 1 diabetes [9, 10]; however, current studies have failed to demonstrate a beneficial effect of intensive diabetes therapy on CVD in type 2 diabetes [11–13].
The development of new classes of blood glucose-lowering medications to supplement the older therapies, such as lifestyle-directed interventions, insulin, sulfonylureas and metformin, has increased the number of treatment options available for type 2 diabetes. Whether used alone or in combination with other blood glucose-lowering interventions, the increased number of choices available to practitioners and patients has heightened uncertainty regarding the most appropriate means of treating this widespread disease [14]. Although numerous reviews on the management of type 2 diabetes have been published in recent years [15–17], practitioners are often left without a clear pathway of therapy to follow. We developed the following consensus approach to the management of hyperglycaemia in the non-pregnant adult to help guide healthcare providers in choosing the most appropriate interventions for their patients with type 2 diabetes.
Process
The guidelines and algorithm that follow are derived from two sources. One source is the clinical trials that address the effectiveness and safety of the different modalities of therapy. Here, the writing group reviewed a wide variety of studies related to the use of drugs as monotherapy or in combination to lower glycaemia. Unfortunately, the paucity of high-quality evidence in the form of well-controlled clinical trials that directly compare different diabetes treatment regimens remains a major impediment to recommending one class of drugs, or a particular combination of therapies, over another.
The second source of material that informed our recommendations was clinical judgement, that is, our collective knowledge and clinical experience, which takes into account benefits, risks and costs in the treatment of diabetes. As in all clinical decision-making, an evidence-based review of the literature must also be supplemented by value judgements, where the benefits of treatment are weighed against risks and costs in a subjective fashion. While we realise that others may have different judgements, we believe that the recommendations made in this new iteration of our treatment algorithm will guide therapy and result in improved glycaemic control and health status over time.
Glycaemic goals of therapy
Controlled clinical trials, such as the DCCT [4] and the Stockholm Diabetes Study in type 1 diabetes [5], and the UK Prospective Diabetes Study (UKPDS) [6, 7] and Kumamoto study [8] in type 2 diabetes, have helped to establish the glycaemic goals of therapy that result in improved long-term outcomes. The clinical trials, in concert with epidemiological data [18, 19], support decreasing glycaemia as an effective means of reducing long-term microvascular and neuropathic complications. The most appropriate target levels for blood glucose, on a day-to-day basis, and HbA1c, as an index of chronic glycaemia, have not been systematically studied. However, both the DCCT [4] and the UKPDS [6, 7] had as their goals the achievement of glycaemic levels in the non-diabetic range. Neither study was able to maintain HbA1c levels in the non-diabetic range in their intensive treatment groups, achieving mean levels over time of ~7%, which is 4 SDs above the non-diabetic mean.
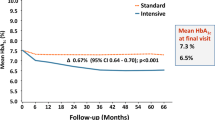
The most recent glycaemic goal recommended by the American Diabetes Association, selected on the basis of practicality and the projected reduction in complications over time, is, in general, an HbA1c level of <7% [1]. The most recent glycaemic goal set by the International Diabetes Federation is an HbA1c level of <6.5%. The upper limit of the non-diabetic range is 6.1% (mean HbA1c level of 5% + 2 SD) with the DCCT/UKPDS-standardised assay, which has been promulgated through the National Glycohemoglobin Standardization Program (NGSP) and adopted by the vast majority of commercially available assays [20]. Several recent clinical trials have aimed for HbA1c levels ≤6.5% with a variety of interventions [11, 12]. The results of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) study, which had the primary objective of decreasing CVD with interventions aimed at achieving an HbA1c level of <6.0% vs interventions aimed at achieving an HbA1c level of <7–7.9%, showed excess CVD mortality in the intensive treatment group [11]. Results from the Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE) trial and the Veterans Affairs Diabetes Trial, both of which had different interventions and study populations than ACCORD, did not demonstrate any excess total or CVD mortality with intensive regimens that achieved HbA1c levels comparable to the 6.5% in ACCORD [12, 13]. However, none of the studies has demonstrated a benefit of intensive glycaemic control on their primary CVD outcomes. Our consensus is that an HbA1c level of ≥7% should serve as a call to action to initiate or change therapy with the goal of achieving an HbA1c level of <7%. We are mindful that this goal is not appropriate or practical for some patients, and clinical judgement based on the potential benefits and risks of a more intensified regimen needs to be applied for every patient. Factors such as life expectancy, risk of hypoglycaemia and the presence of CVD need to be considered for every patient before intensifying the therapeutic regimen.
Assiduous attention to abnormalities other than hyperglycaemia that accompany type 2 diabetes, such as hypertension and dyslipidaemia, has been shown to improve microvascular and cardiovascular complications. Readers are referred to published guidelines for a discussion of the rationale and goals of therapy for the non-glycaemic risk factors, as well as recommendations on how to achieve them [1, 21, 22].
Principles in selecting antihyperglycaemic interventions
Our choice of specific antihyperglycaemic agents is predicated on their effectiveness in lowering glucose, extraglycaemic effects that may reduce long-term complications, safety profiles, tolerability, ease-of-use and expense.
Effectiveness in lowering glycaemia
Except for their differential effects on glycaemia, there are insufficient data at this time to support a recommendation of one class of glucose-lowering agents, or one combination of medications, over others with regard to effects on complications. In other words, the salutary effects of therapy on long-term complications appear to be predicated predominantly on the level of glycaemic control achieved, rather than on any other specific attributes of the intervention(s) used to achieve glycaemic goals. The UKPDS compared three classes of glucose-lowering medications (sulfonylurea, metformin or insulin), but was unable to demonstrate clear superiority of any one drug over the others with regard to diabetic complications [6, 7]. However, the different classes do have variable effectiveness in decreasing glycaemic levels (Table 1), and the overarching principle in selecting a particular intervention will be its ability to achieve and maintain glycaemic goals. In addition to their intention-to-treat analyses demonstrating the superiority of intensive vs conventional interventions, the DCCT and UKPDS demonstrated a strong correlation between mean HbA1c levels over time and the development and progression of retinopathy and nephropathy [23, 24]. Therefore, we think it is reasonable to judge and compare blood glucose-lowering medications, and combinations of such agents, primarily on the basis of their capacity to decrease and maintain HbA1c levels and according to their safety, specific side effects, tolerability, ease-of-use and expense.
Non-glycaemic effects of medications
In addition to variable effects on glycaemia, specific effects of individual therapies on CVD risk factors, such as hypertension or dyslipidaemia, were also considered important. We also included the effects of interventions that may benefit or worsen the prospects for long-term glycaemic control in our recommendations. Examples of these would be changes in body mass, insulin resistance, or insulin secretory capacity in type 2 diabetic patients.
Choosing specific diabetes interventions and their roles in treating type 2 diabetes
Numerous reviews have focused on the characteristics of the specific diabetes interventions listed below [25–34]. In addition, meta-analyses and reviews have summarised and compared the glucose-lowering effectiveness and other characteristics of the medications [35–37]. The aim here is to provide enough information to justify the choices of medications, the order in which they are recommended and the use of combinations of therapies. Unfortunately, there is a dearth of high-quality studies that provide head-to-head comparisons of the ability of the medications to achieve the currently recommended glycaemic levels. The authors highly recommend that such studies be conducted. However, even in the absence of rigorous, comprehensive studies that directly compare the efficacy of all available glucose-lowering treatments and their combinations, we feel that there are enough data regarding the characteristics of the individual interventions to provide the guidelines below.
An important intervention that is likely to improve the probability that a patient will have better long-term control of diabetes is to make the diagnosis early, when the metabolic abnormalities of diabetes are usually less severe. Lower levels of glycaemia at the time of initial therapy are associated with lower HbA1c levels over time and decreased long-term complications [38].
Lifestyle interventions
The major environmental factors that increase the risk of type 2 diabetes are overnutrition and a sedentary lifestyle, with consequent overweight and obesity [39, 40]. Not surprisingly, interventions that reverse or improve these factors have been demonstrated to have a beneficial effect on control of glycaemia in established type 2 diabetes [41]. Unfortunately, the high rate of weight regain has limited the role of lifestyle interventions as an effective means of controlling glycaemia in the long term. The most convincing long-term data indicating that weight loss effectively lowers glycaemia have been generated in the follow-up of type 2 diabetic patients who have had bariatric surgery. In this setting, with a mean sustained weight loss of >20 kg, diabetes is virtually eliminated [42–45]. In addition to the beneficial effects of weight loss on glycaemia, weight loss and exercise improve coincident CVD risk factors, such as blood pressure and atherogenic lipid profiles, and ameliorate other consequences of obesity [41, 46, 47]. There are few adverse consequences of such lifestyle interventions other than difficulty in incorporating them into usual lifestyle and sustaining them, and the usually minor musculoskeletal injuries and potential problems associated with neuropathy, such as foot trauma and ulcers, that may occur as a result of increased activity. Theoretically, effective weight loss, with its pleiotropic benefits, safety profile and low cost, should be the most cost-effective means of controlling diabetes, if it could be achieved and maintained over the long term.
Given these beneficial effects, which are usually seen rapidly—within weeks to months—and often before there has been substantial weight loss [47], a lifestyle intervention programme to promote weight loss and increase activity levels should, with rare exceptions, be included as part of diabetes management. Weight loss of as little as 4 kg will often ameliorate hyperglycaemia. However, the limited long-term success of lifestyle programmes to maintain glycaemic goals in patients with type 2 diabetes suggests that the large majority of patients will require the addition of medications over the course of their diabetes.
Medications
The characteristics of currently available glucose-lowering interventions, when used as monotherapy, are summarised in Table 1. The glucose-lowering effectiveness of individual therapies and combinations demonstrated in clinical trials is predicated not only on the intrinsic characteristics of the intervention, but also on the duration of diabetes, baseline glycaemia, previous therapy and other factors. A major factor in selecting a class of drugs, or a specific medication within a class, to initiate therapy or when changing therapy, is the ambient level of glycaemic control. When levels of glycaemia are high (e.g. HbA1c > 8.5%), classes with greater and more rapid glucose-lowering effectiveness, or potentially earlier initiation of combination therapy, are recommended; however, patients with recent-onset diabetes often respond adequately to less intensive interventions than those with longer term disease [48]. When glycaemic levels are closer to the target levels (e.g. HbA1c < 7.5%), medications with lesser potential to lower glycaemia and/or a slower onset of action may be considered.
Obviously, the choice of glycaemic goals and the medications used to achieve them must be individualised for each patient, balancing the potential for lowering HbA1c and anticipated long-term benefit with specific safety issues, as well as other characteristics of regimens, including side effects, tolerability, ease-of-use, long-term adherence, expense and the non-glycaemic effects of the medications. Type 2 diabetes is a progressive disease characterised by worsening glycaemia; higher doses and additional medications are required over time if treatment goals are to be met.
Metformin
In most of the world, metformin is the only biguanide available. Its major effect is to decrease hepatic glucose output and lower fasting glycaemia. Typically, metformin monotherapy will lower HbA1c levels by ~1.5 percentage points [27, 49]. It is generally well tolerated, with the most common adverse effects being gastrointestinal. Metformin monotherapy is not usually accompanied by hypoglycaemia, and has been used safely, without causing hypoglycaemia, in patients with pre-diabetic hyperglycaemia [50]. Metformin interferes with vitamin B12 absorption, but is very rarely associated with anaemia [27]. The major non-glycaemic effect of metformin is either weight stability or modest weight loss, in contrast to many of the other blood glucose-lowering medications. The UKPDS demonstrated a beneficial effect of metformin therapy on CVD outcomes [7], which needs to be confirmed. Renal dysfunction is considered a contraindication to metformin use because it may increase the risk of lactic acidosis, an extremely rare (less than one case per 100,000 treated patients) but potentially fatal complication [51]. However, recent studies have suggested that metformin is safe unless the estimated glomerular filtration rate falls to <30 ml/min [52].
Sulfonylureas
Sulfonylureas lower glycaemia by enhancing insulin secretion. In terms of efficacy, they appear to be similar to metformin, lowering HbA1c levels by ~1.5 percentage points [26, 49]. The major adverse side effect is hypoglycaemia, which can be prolonged and life-threatening, but such episodes, characterised by a need for assistance, coma or seizure, are infrequent. However, severe episodes are relatively more frequent in the elderly. Chlorpropamide and glibenclamide (known as glyburide in the USA and Canada), are associated with a substantially greater risk of hypoglycaemia than other second-generation sulfonylureas (gliclazide, glimepiride, glipizide, and their extended formulations), which are preferable (Table 1) [53, 54]. In addition, weight gain of ~2 kg is common following the initiation of sulfonylurea therapy. Although the onset of the glucose-lowering effect of sulfonylurea monotherapy is relatively rapid compared with, for example, the thiazolidinediones (TZDs), maintenance of glycaemic targets over time is not as good as monotherapy with a TZD or metformin [55]. Sulfonylurea therapy was implicated as a potential cause of increased CVD mortality in the University Group Diabetes Program (UGDP) study [56]. Concerns raised by the UGDP that sulfonylureas, as a drug class, may increase CVD mortality in type 2 diabetes were not substantiated by the UKPDS or ADVANCE study [6, 12]. The glycaemic benefits of sulfonylureas are nearly fully realised at half-maximal doses and higher doses should generally be avoided.
Glinides
Like the sulfonylureas, the glinides stimulate insulin secretion, although they bind to a different site within the sulfonylurea receptor [28]. They have a shorter circulating half-life than the sulfonylureas and must be administered more frequently. Of the two glinides currently available in the USA, repaglinide is almost as effective as metformin or the sulfonylureas, decreasing HbA1c levels by ~1.5 percentage points. Nateglinide is somewhat less effective in lowering HbA1c than repaglinide when used as monotherapy or in combination therapy [57, 58]. The risk of weight gain is similar to that for the sulfonylureas, but hypoglycaemia may be less frequent, at least with nateglinide, than with some sulfonylureas [58, 59].
α-Glucosidase inhibitors
α-Glucosidase inhibitors reduce the rate of digestion of polysaccharides in the proximal small intestine, primarily lowering postprandial glucose levels without causing hypoglycaemia. They are less effective in lowering glycaemia than metformin or the sulfonylureas, reducing HbA1c levels by 0.5–0.8 percentage points [29]. Since carbohydrate is absorbed more distally, malabsorption and weight loss do not occur; however, increased delivery of carbohydrate to the colon commonly results in increased gas production and gastrointestinal symptoms. In clinical trials, 25–45% of participants have discontinued α-glucosidase inhibitor use as a result of this side effect [29, 60].
One clinical trial examining acarbose as a means of preventing the development of diabetes in high-risk individuals with impaired glucose tolerance showed an unexpected reduction in severe CVD outcomes [60]. This potential benefit of α-glucosidase inhibitors needs to be confirmed.
Thiazolidinediones
Thiazolidinediones (TZDs or glitazones) are peroxisome proliferator-activated receptor γ modulators; they increase the sensitivity of muscle, fat and liver to endogenous and exogenous insulin (‘insulin sensitisers’) [31]. The data regarding the blood glucose-lowering effectiveness of TZDs when used as monotherapy have demonstrated a 0.5–1.4 percentage point decrease in HbA1c. The TZDs appear to have a more durable effect on glycaemic control, particularly compared with sulfonylureas [55]. The most common adverse effects with TZDs are weight gain and fluid retention, with peripheral oedema and a twofold increased risk for congestive heart failure [61, 62]. There is an increase in adiposity, largely subcutaneous, with some reduction in visceral fat shown in some studies. The TZDs either have a beneficial (pioglitazone) or neutral (rosiglitazone) effect on atherogenic lipid profiles [63, 64]. Several meta-analyses have suggested a 30–40% relative increase in risk for myocardial infarction [65, 66] with rosiglitazone. On the other hand, the Prospective Pioglitazone Clinical Trial in macrovascular events (PROactive) demonstrated no significant effects of pioglitazone compared with placebo on the primary CVD outcome (a composite of all-cause mortality, non-fatal and silent myocardial infarction, stroke, major leg amputation, acute coronary syndrome, coronary artery bypass graft or percutaneous coronary intervention, and leg revascularisation) after 3 years of follow-up [67]. Pioglitazone was associated with a 16% reduction in death, myocardial infarction and stroke—a controversial secondary endpoint reported to have marginal statistical significance [67]. Meta-analyses have supported a possible beneficial effect of pioglitazone on CVD risk [68]. Although the data are less than conclusive for a CVD risk with rosiglitazone or a CVD benefit with pioglitazone, we have previously advised [69] caution in using either TZD, on the basis that they are both associated with increased risks of fluid retention and congestive heart failure, and an increased incidence of fractures in women, and perhaps in men [55, 61, 62, 70]. Although the meta-analyses discussed above are not conclusive regarding the potential cardiovascular risk associated with rosiglitazone, given that other options are now recommended, the consensus group members unanimously advised against using rosiglitazone. Currently, in the USA, the TZDs are approved for use in combination with metformin, sulfonylureas, glinides and insulin.
Insulin
Insulin is the oldest of the currently available medications, and therefore the treatment with which we have the most clinical experience. It is also the most effective at lowering glycaemia. Insulin can, when used in adequate doses, decrease any level of elevated HbA1c to, or close to, the therapeutic goal. Unlike the other blood glucose-lowering medications, there is no maximum dose of insulin beyond which a therapeutic effect will not occur. Relatively large doses of insulin (≥1 U/kg), compared with those required to treat type 1 diabetes, may be necessary to overcome the insulin resistance of type 2 diabetes and lower HbA1c to the target level. Although initial therapy is aimed at increasing basal insulin supply, usually with intermediate- or long-acting-insulins, patients may also require prandial therapy with short- or rapid-acting insulins (Fig. 1). The very rapid-acting and long-acting insulin analogues have not been shown to lower HbA1c levels more effectively than the older, rapid-acting or intermediate-acting formulations [71–73]. Insulin therapy has beneficial effects on triacylglycerol and HDL-cholesterol levels, especially in patients with poor glycaemic control [74], but is associated with weight gain of ~2–4 kg, which is probably proportional to the correction of glycaemia and predominantly the result of the reduction of glycosuria. Insulin therapy is also associated with hypoglycaemia, albeit much less frequently than in type 1 diabetes. In clinical trials aimed at normoglycaemia and achieving a mean HbA1c of ~7%, severe hypoglycaemic episodes (defined as requiring help from another person to treat) occurred at a rate of between one and three per 100 patient-years [8, 75–77], compared with 61 per 100 patient-years in the DCCT intensive therapy group [4]. Insulin analogues with longer, non-peaking profiles decrease the risk of hypoglycaemia modestly compared with NPH, and analogues with very short durations of action reduce the risk of hypoglycaemia compared with regular insulin [76, 77].
Initiation and adjustment of insulin regimens. Insulin regimens should be designed taking lifestyle and meal schedule into account. The algorithm can only provide basic guidelines for initiation and adjustment of insulin. See reference 90 for more detailed instructions. aPre-mixed insulins not recommended during adjustment of doses; however, they can be used conveniently, usually before breakfast and/or dinner, if proportion of rapid and intermediate-acting insulins is similar to the fixed proportions available. bg, blood glucose
Glucagon-like peptide 1 agonists (exenatide)
Glucagon-like peptide 1 (GLP-1) 7–37, a naturally occurring peptide produced by the L cells of the small intestine, potentiates glucose-stimulated insulin secretion. Exendin-4 has homology with the human GLP-1 sequence, but has a longer circulating half-life. It binds avidly to the GLP-1 receptor on the pancreatic beta cell and augments glucose-mediated insulin secretion [32]. Synthetic exendin-4 (exenatide) was approved for use in the USA in 2005 and is administered twice per day by subcutaneous injection. Although there are less published data on this new compound than the other blood glucose-lowering medications, exendin-4 appears to lower HbA1c levels by 0.5–1 percentage points, mainly by lowering postprandial blood glucose levels [78–81]. Exenatide also suppresses glucagon secretion and slows gastric motility. It is not associated with hypoglycaemia, but causes a relatively high frequency of gastrointestinal disturbances, with 30–45% of treated patients experiencing one or more episodes of nausea, vomiting or diarrhoea [78–81]. These side effects tend to abate over time. In published trials, exenatide is associated with weight loss of ~2–3 kg over 6 months, some of which may be a result of its gastrointestinal side effects. Recent reports have suggested a risk for pancreatitis associated with use of GLP agonists; however, the number of cases is very small and whether the relationship is causal or coincidental is not clear at this time. Currently, exenatide is approved for use in the USA with sulfonylurea, metformin and/or a TZD. Several other GLP-1 agonists and formulations are under development.
Amylin agonists (pramlintide)
Pramlintide is a synthetic analogue of the beta cell hormone amylin. It is administered subcutaneously before meals and slows gastric emptying, inhibits glucagon production in a glucose-dependent fashion, and predominantly decreases postprandial glucose excursions [33]. In clinical studies, HbA1c has been decreased by 0.5–0.7 percentage points [82]. The major clinical side effects of this drug are gastrointestinal in nature. Approximately 30% of treated participants in the clinical trials have developed nausea, but this side effect tends to abate with time on therapy. Weight loss associated with this medication is ~1–1.5 kg over 6 months; as with exenatide, some of the weight loss may be the result of gastrointestinal side effects. Currently, pramlintide is approved for use in the USA only, as adjunctive therapy with regular insulin or rapid-acting insulin analogues.
Dipeptidyl peptidase 4 inhibitors
GLP-1 and glucose-dependent insulinotropic peptide (GIP), the main insulinotropic peptides of intestinal origin (incretins), are rapidly degraded by dipeptidyl peptidase 4 (DPP-4). DPP-4 is a member of a family of cell membrane proteins that are expressed in many tissues, including immune cells [34]. DPP-4 inhibitors are small molecules that enhance the effects of GLP-1 and GIP, increasing glucose-mediated insulin secretion and suppressing glucagon secretion. [83, 84]. The first oral DPP-4 inhibitor, sitagliptin, was approved by the Food and Drug Administration in October 2006 for use as monotherapy or in combination with metformin or TZDs. Another DPP-4 inhibitor, vildagliptin, was approved in Europe in February 2008, and several other compounds are under development. In clinical trials performed to date, DPP-4 inhibitors lower HbA1c levels by 0.6–0.9 percentage points and are weight neutral and relatively well tolerated [83, 84]. They do not cause hypoglycaemia when used as monotherapy. A fixed-dose combination pill with metformin is available. The potential for this class of compounds to interfere with immune function is of concern; an increase in upper respiratory infections has been reported [34].
How to initiate diabetes therapy and advance interventions
Except in rare circumstances, such as diabetic ketoacidosis or patients who are extremely catabolic or hyperosmolar or who are unable to hydrate themselves adequately (see Special considerations/patients below), hospitalisation is not required for initiation or adjustment of therapy. The patient is the key player in the diabetes care team and should be trained and empowered to adjust medications with the guidance of healthcare professionals to achieve glycaemic goals and to prevent and treat hypoglycaemia. Many patients may be managed effectively with monotherapy; however, the progressive nature of the disease will require the use of combination therapy in many, if not most, patients over time, to achieve and maintain glycaemia in the target range.
The measures of glycaemia that are initially targeted on a day-to-day basis are fasting and preprandial glucose levels. Self-monitoring of blood glucose (SMBG) is an important element in adjusting or adding new interventions and, in particular, in titrating insulin doses. The need for and number of required SMBG measurements are not clear [85] and are dependent on the medications used. Oral glucose-lowering regimens that do not include sulfonylureas or glinides, and are therefore not likely to cause hypoglycaemia, usually do not require SMBG. [86] However, SMBG may be used to determine whether therapeutic blood glucose targets are being achieved and for adjustment of treatment regimens without requiring the patient to have laboratory-based blood glucose testing. Insulin therapy requires more frequent monitoring.
The levels of plasma or capillary glucose (most meters that measure fingerstick capillary samples are adjusted to provide values equivalent to plasma glucose) that should result in long-term glycaemia in the non-diabetic target range, as measured by HbA1c, are fasting and preprandial levels between 3.9 and 7.2 mmol/l (70 and 130 mg/dl). If HbA1c levels remain above the desired target despite preprandial levels that are in range, postprandial levels, usually measured 90–120 min after a meal, may be checked. They should be <10 mmol/l (180 mg/dl) to achieve HbA1c levels in the target range.
Attempts to achieve target glycaemic levels with regimens including sulfonylureas or insulin may be associated with modest hypoglycaemia, with glucose levels in the 3.1–3.9 mmol/l (55–70 mg/dl) range. These episodes are generally well tolerated, easily treated with oral carbohydrate, such as glucose tablets or 120–180 ml (4–6 oz) of juice or non-diet soda, and rarely progress to more severe hypoglycaemia, including loss of consciousness or seizures.
Algorithm
The algorithm (Fig. 2) takes into account the characteristics of the individual interventions, their synergies and expense. The goal is to achieve and maintain HbA1c levels of <7% and to change interventions at as rapid a pace as titration of medications allows when target glycaemic goals are not being achieved. Mounting evidence suggests that aggressive lowering of glycaemia, especially with insulin therapy, in newly diagnosed diabetes can result in sustained remissions, i.e. normoglycaemia without need for glucose-lowering medications [87, 88]. Type 2 diabetes is a progressive disease [89] and patients should be informed that they are likely to require the addition of glucose-lowering medications over time.
Algorithm for the metabolic management of type 2 diabetes. Reinforce lifestyle interventions at every visit. Check HbA1c every 3 months until HbA1c is <7%, and then at least every 6 months. The interventions should be changed if HbA1c is ≥7%. aSulfonylureas other than glybenclamide (glyburide) or chlorpropamide. bInsufficient clinical use to be confident regarding safety. See text box: Titration of metformin. See Fig. 1 for initiation and adjustment of insulin. CHF, congestive heart failure
The amylin agonists, α-glucosidase inhibitors, glinides, and DPP-4 inhibitors are not included in the two tiers of preferred agents in this algorithm, owing to their lower or equivalent overall glucose-lowering effectiveness compared with the first and second tier agents and/or to their limited clinical data or relative expense (Table 1). However, they may be appropriate choices in selected patients.
-
Tier 1:
well-validated core therapies
These interventions represent the best established and most effective and cost-effective therapeutic strategy for achieving the target glycaemic goals. The tier 1 algorithm is the preferred route of therapy for most patients with type 2 diabetes.
Step 1: lifestyle intervention and metformin
Based on the numerous demonstrated short- and long-term benefits that accrue when weight loss and increased levels of activity are achieved and maintained, and the cost-effectiveness of lifestyle interventions when they succeed, the consensus is that lifestyle interventions should be initiated as the first step in treating new-onset type 2 diabetes (Fig. 2). These interventions should be implemented by healthcare professionals with appropriate training, usually registered dietitians experienced in behavioural modification, and be sensitive to ethnic and cultural differences among populations. Moreover, lifestyle interventions to improve glucose, blood pressure and lipid levels, and to promote weight loss or at least avoid weight gain, should remain an underlying theme throughout the management of type 2 diabetes, even after medications are used. For the 10–20% of patients with type 2 diabetes who are not obese or overweight, modification of dietary composition and activity levels may play a supporting role, but medications are still generally required early in the course of diabetes (see Special considerations/patients below).
The authors recognise that for most persons with type 2 diabetes, lifestyle interventions fail to achieve or maintain the metabolic goals, either because of failure to lose weight, weight regain, progressive disease or a combination of factors. Therefore, our consensus is that metformin therapy should be initiated concurrently with lifestyle intervention at diagnosis. Metformin is recommended as the initial pharmacological therapy, in the absence of specific contraindications, for its effect on glycaemia, absence of weight gain or hypoglycaemia, generally low level of side effects, high level of acceptance and relatively low cost. Metformin treatment should be titrated to its maximally effective dose over 1–2 months, as tolerated (see text box: Titration of metformin). Rapid addition of other glucose-lowering medications should be considered in the setting of persistent symptomatic hyperglycaemia.
Step 2: addition of a second medication
If lifestyle intervention and the maximal tolerated dose of metformin fail to achieve or sustain the glycaemic goals, another medication should be added within 2–3 months of the initiation of therapy or at any time when the target HbA1c level is not achieved. Another medication may also be necessary if metformin is contraindicated or not tolerated. The consensus regarding the second medication added to metformin was to choose either insulin or a sulfonylurea (Fig. 2). As discussed above, the HbA1c level will determine in part which agent is selected next, with consideration given to the more effective glycaemia-lowering agent, insulin, for patients with an HbA1c level of >8.5% or with symptoms secondary to hyperglycaemia. Insulin can be initiated with a basal (intermediate- or long-acting) insulin (see Fig. 1 for suggested initial insulin regimens) [90]. However, many newly diagnosed type 2 diabetic patients will usually respond to oral medications, even if symptoms of hyperglycaemia are present [48].
Step 3: further adjustments
If lifestyle, metformin and sulfonylurea or basal insulin do not result in achievement of target glycaemia, the next step should be to start, or intensify, insulin therapy (Fig. 1). Intensification of insulin therapy usually consists of additional injections that might include a short- or rapid-acting insulin given before selected meals to reduce postprandial glucose excursions (Fig. 1). When insulin injections are started, insulin secretagogues (sulfonylurea or glinides) should be discontinued, or tapered and then discontinued, since they are not considered to be synergistic. Although addition of a third oral agent can be considered, especially if the HbA1c level is close to target (HbA1c <8.0%), this approach is usually not preferred as it is no more effective in lowering glycaemia, and is more costly, than initiating or intensifying insulin [91].
-
Tier 2:
less well-validated therapies
In selected clinical settings, this second tier algorithm may be considered. Specifically, when hypoglycaemia is particularly undesirable (for example, in patients who have hazardous jobs), the addition of exenatide or pioglitazone may be considered. Rosiglitazone is not recommended. If promotion of weight loss is a major consideration and the HbA1c level is close to target (<8.0%), exenatide is an option. If these interventions are not effective in achieving target HbA1c, or are not tolerated, addition of a sulfonylurea could be considered. Alternatively, the tier 2 interventions should be stopped and basal insulin started.
Rationale for selecting specific combinations
More than one medication will be necessary for the majority of patients over time. Selection of the individual agents should be made on the basis of their glucose-lowering effectiveness and other characteristics listed in Table 1. However, when adding second antihyperglycaemic medications, the synergy of particular combinations and other interactions should be considered. In general, antihyperglycaemic drugs with different mechanisms of action will have the greatest synergy. Insulin plus metformin [92] is a particularly effective means of lowering glycaemia while limiting weight gain.
Special considerations/patients
In the setting of severely uncontrolled diabetes with catabolism, defined as fasting plasma glucose levels >13.9 mmol/l (250 mg/dl), random glucose levels consistently >16.7 mmol/l (300 mg/dl), HbA1c >10% or the presence of ketonuria, or as symptomatic diabetes with polyuria, polydipsia and weight loss, insulin therapy in combination with lifestyle intervention is the treatment of choice. Some patients with these characteristics will have unrecognised type 1 diabetes; others will have type 2 diabetes with severe insulin deficiency. Insulin can be titrated rapidly and is associated with the greatest likelihood of returning glucose levels rapidly to target levels. After symptoms are relieved and glucose levels decreased, oral agents can often be added and it may be possible to withdraw insulin, if preferred.
Conclusions
Type 2 diabetes is epidemic. Its long-term consequences translate into enormous human suffering and economic costs; however, much of the morbidity associated with long-term microvascular and neuropathic complications can be substantially reduced by interventions that achieve glucose levels close to the non-diabetic range. Although new classes of medications, and numerous combinations, have been demonstrated to lower glycaemia, current-day management has failed to achieve and maintain the glycaemic levels most likely to provide optimal healthcare status for people with diabetes.


Duality of interest
D. M. Nathan has received a research grant for investigator-initiated research from Sanofi Aventis and support for educational programs from GlaxoSmithKline. J. B. Buse has conducted research and/or served on advisory boards under contract between the University of North Carolina and Amylin, Becton-Dickinson, Bristol-Myers Squibb, Hoffman-LaRoche, Eli Lilly, GlaxoSmithKline, Novo Nordisk, Merck, Novartis, Pfizer and Sanofi Aventis. M. B. Davidson has received research support from Eli Lilly, Merck and Pfizer; has served on advisory boards for Amylin, GlaxoSmithKline, Merck and Sanofi Aventis; and has been on speakers bureaus for Amylin, Eli Lilly, GlaxoSmithKline and Pfizer. E. Ferrannini has received research support from AstraZeneca, Merck Sharpe & Dohme and Novartis and serves on scientific advisory boards for Amylin, AstraZeneca, GlaxoSmithKline, Roche, Merck Sharpe & Dohme, Novartis, Sanofi Aventis, Boehringer Ingelheim and Takeda. R. R. Holman has received research support from Bristol-Myers Squibb, GlaxoSmithKline, Merck Sante, Novo Nordisk, Pfizer and Pronova and has served on advisory boards and/or received honoraria for speaking engagements from Amylin, GlaxoSmithKline, Lilly, Merck Sharp & Dohme, Novartis and Sanofi Aventis. R. Sherwin has served on advisory boards for Amylin, AstraZeneca, Boehringer Ingelheim, DiObex, Eli Lilly, Insulet, Merck, MannKind and Novartis. B. Zinman has received research support from GlaxoSmithKline, Merck, Novartis and Novo Nordisk and has been a member of scientific advisory boards and /or received honoraria for speaking engagements from Amylin, Eli Lilly, GlaxoSmithKline, Merck, Novartis, Pfizer, Sanofi Aventis and Servier.
Abbreviations
- ACCORD:
-
Action to Control Cardiovascular Risk in Diabetes
- ADVANCE:
-
Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation
- CVD:
-
cardiovascular disease
- DPP-4:
-
dipeptidyl peptidase 4
- GIP:
-
glucose-dependent insulinotropic peptide
- GLP-1:
-
glucagon-like peptide 1
- SMBG:
-
self-monitoring of blood glucose
- TZD:
-
thiazolidinedione
- UGDP:
-
University Group Diabetes Program
- UKPDS:
-
UK Prospective Diabetes Study
References
American Diabetes Association. Standards of medical care in diabetes (2008) Diabetes Care 31(Suppl 1):S12–S54
European Diabetes Policy Group (1999) A desk-top guide to type 2 diabetes mellitus. Diabet Med 16:716–730
National Institute for Clinical Excellence (2002) Clinical guidelines for type 2 diabetes mellitus: management of blood glucose. London, NICE. Available from http://www.nice.org.uk/Guidance/CG66
Diabetes Control and Complications Trial Research Group (1993) The effect of intensive diabetes treatment on the development and progression of long-term complications in insulin-dependent diabetes mellitus: the Diabetes Control and Complications Trial. N Engl J Med 329:978–986
Reichard P, Nilsson B-Y, Rosenqvist U (1993) The effect of long-term intensified insulin treatment on the development of microvascular complications of diabetes mellitus. N Engl J Med 329:304–309
UK Prospective Diabetes Study (UKPDS) Group (1998) Intensive blood glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complication in patients with type 2 diabetes (UKPDS 33). Lancet 352:837–853
UK Prospective Diabetes Study (UKPDS) Group (1998) Effect of intensive blood glucose control with metformin on complication in overweight patients with type 2 diabetes (UKPDS 34). Lancet 352:854–865
Ohkubo Y, Kishikawa H, Araki E et al (1995) Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with NIDDM: A randomized prospective 6-year study. Diab Res Clin Pract 28:103–117
Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group (2003) Intensive diabetes therapy and carotid intima–media thickness in type 1 diabetes. N Engl J Med 348:2294–2303
Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group (2005) Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Eng J Med 353:2643–2653
The Action to Control Cardiovascular Risk in Diabetes Study Group (2008) Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 358:2545–2559
The ADVANCE Collaborative Group (2008) Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 358:2560–2572
Abraira C, Duckworth WC, Moritz T (2008) Glycaemic separation and risk factor control in the Veterans Affairs Diabetes Trial: an interim report. Diabetes Obes Metab. doi:10.1111/j.1463-1326.2008.00933.x
Nathan DM (2007) Finding new treatments for diabetes—how many, how fast … how good? N Engl J Med 356:437–440
Nathan DM (2002) Initial management of glycemia in type 2 diabetes mellitus. N Engl J Med 347:1342–1349
Sheehan MT (2003) Current therapeutic options in type 2 diabetes mellitus: a practical approach. Clin Med Res 1:189–200
Inzucchi SE (2002) Oral antihyperglycemic therapy for type 2 diabetes. JAMA 287:360–72
Klein R, Klein BEK, Moss SE, Davis MD, DeMets DL (1988) Glycosylated hemoglobin predicts the incidence and progression of diabetic retinopathy. JAMA 260:2864–2871
Chase HP, Jackson WE, Hoops SL, Cockerham RS, Archer PG, O’Brien D (1989) Glucose control and the renal and retinal complications of insulin-dependent diabetes. JAMA 261:1155–60
Little RR, Rohlfing CL, Wiedmeyer H-M, Myers GL, Sacks DB, Goldstein DE (2001) The National Glycohemoglobin Standardization Program (NGSP): a five year progress report. Clin Chem 47:1985–1992
Grundy SM, Cleeman JI, Merz NB et al (2004) Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation 110:227–239
Chobanian AV, Bakris GL, Black HR et al (2003) National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation and Treatment Of High Blood Pressure; National High blood Pressure Education Program Coordinating committee. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment Of High Blood Pressure: the JNC 7 report. JAMA 289:2560–2572
DCCT Research Group (1995) The association between glycemic exposure and long-term diabetic complications in the Diabetes Control and Complications Trial. Diabetes 44:968–983
Stratton IM, Adler AI, Neil HA et al (2000) Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 321:405–412
National Institutes of Health (1999) Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. The Evidence Report. National Heart, Lung, and Blood Institute, NIH, Bethesda, MD
Groop L (1992) Sulfonylureas in NIDDM. Diabetes Care 15:737–747
Bailey CJ, Turner RC (1996) Metformin. N Engl J Med 334:574–583
Malaisse WJ (2003) Pharmacology of the meglitinide analogs: new treatment options for type 2 diabetes mellitus. Treat Endocrinol 2:401–414
Van de Laar FA, Lucassen PL, Akkermans RP et al (2005) Alpha-glucosidase inhibitors for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2:CD003639
Genuth S (1990) Insulin use in NIDDM. Diabetes Care 13:1240–64
Yki-Jarvinen H (2004) Drug therapy: thiazolidinediones. N Engl J Med 351:1106
Drucker DJ (2005) Biologic actions and therapeutic potential of the proglucagon-derived peptides. Nature Endocrinol Metab 1:22–31
Schmitz O, Brock B, Rungby J (2004) Amylin agonists: a novel approach in the treatment of diabetes. Diabetes 53(Suppl 3):S233–238
Richter B, Bandeira-Echtler E, Bergerhoff K, Lerch C (2008) Dipeptidyl peptidase-4 (DPP-4) inhibitors for type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2:CD006739
Amori RE, Lau J, Pittas AG (2007) Efficacy and safety of incretin therapy in type 2 diabetes: a systematic review and meta-analysis. JAMA 298:194–206
Monami M, Lamannac C, Marchionni N, Mannucci E (2008) Comparison of different drugs as add-on treatments to metformin in type 2 diabetes: a meta-analysis. Diabetes Res Clin Pract 79:196–203
Bolen S, Feldman L, Vassy J et al (2007) Systematic review: comparative effectiveness and safety of oral medications for type 2 diabetes mellitus. Ann Intern Med 147:386–399
Colagiuri S, Cull CA, Holman RR, UKPDS Group (2002) Are lower fasting plasma glucose levels at diagnosis of type 2 diabetes associated with improved outcomes?: UK Prospective Diabetes Study 61. Diabetes Care 25:1410–1417
Harris MI (1991) Epidemiologic correlates of NIDDM in Hispanics, whites and blacks in the U.S. population. Diabetes Care 14(Suppl 3):639–648
Rewers M, Hamman RF (1995) Risk factors for non-insulin dependent diabetes. In: Harris M (ed) Diabetes in America, 2nd ed. NIH Publication No. 95–1468. National Institutes of Health, Bethesda, pp 179–220
Look AHEAD Research Group (2007) Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the Look AHEAD trial. Diabetes Care 30:1374–1383
Pories WJ, Swanson MS, MacDonald KG et al (1995) Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg 222:339–350
Sjostrom L, Lindroos AK, Peltonen M, Swedish Obese Subjects Study Scientific Group et al (2004) Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 351:2683–2693
Dixon JB, O’Brien PE, Playfair J et al (2008) Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA 299:316–323
Pontiroli AE, Folli F, Paganelli M et al (2005) Laparoscopic gastric banding prevents type 2 diabetes and arterial hypertension and induces their remission in morbid obesity: a 4-year case–controlled study. Diabetes Care 28:2703–2709
Diabetes Prevention Program Research Group (2005) Impact of intensive lifestyle and metformin therapy on cardiovascular disease risk factors in the Diabetes Prevention Program. Diabetes Care 28:888–894
Hadden DR, Montgomery DAD, Skelly RJ et al (1975) Maturity onset diabetes mellitus: response to intensive dietary management. BMJ 3:276–278
Peters AL, Davidson MB (1996) Maximal dose glyburide in markedly symptomatic patients with type 2 diabetes: a new use for an old friend. J Clin Endocrinol Metab 81:2423
DeFronzo R, Goodman A (1995) Efficacy of metformin in patients with non-insulin-dependent diabetes mellitus. The Multicenter Metformin Study Group. N Engl J Med 333:541–549
Diabetes Prevention Program Research Group (2002) Reduction in incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 346:393–403
Salpeter S, Greyber E, Pasternak G, Salpeter E (2006) Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database Syst. Rev. 1:CD002967
Shaw JS, Wilmot RL, Kilpatrick ES (2007) Establishing pragmatic estimated GFR thresholds to guide metformin prescribing. Diabetic Medicine 24:1160–1163
Holstein A, Plaschke A, Egberts E-H (2001) Lower incidence of severe hypoglycemia in patients with type 2 diabetes treated with glimepiride versus glibenclamide. Diabetes Metab Res Rev 17:467–473
Gangji AS, Cukierman T, Gerstein HC, Goldsmith CH, Clase CM (2007) A systematic review and meta-analysis of hypoglycemia and cardiovascular events: a comparison of glyburide with other secretagogues and with insulin. Diabetes Care 30:389–94
Kahn SE, Haffner SM, Heise MA, ADOPT Study Group et al (2006) Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 355:2427–2443
Meinert CL, Knatterud GL, Prout TE, Klimt CR (1970) The University Group Diabetes Program: a study of the effect of hypoglycemic agents on vascular complications in patients with adult-onset diabetes. II Mortality results. Diabetes 19(Suppl 1):789–830
Rosenstock J, Hassman DR, Madder RD et al (2004) Repaglinide versus nateglinide monotherapy: a randomized, multicenter study. Diabetes Care 27:1265–1270
Gerich J, Raskin P, Jean-Louis L, Purkayastha D, Baron A (2005) PRESERVE-beta: two year efficacy and safety of initial combination therapy with nateglinide or glyburide plus metformin. Diabetes Care 28:2093–2099
Damsbo P, Clauson P, Marbury TC, Windfeld K (1999) A double blind randomized comparison of meal-related glycemic control by repaglinide and glyburide in well-controlled type 2 diabetic patients. Diabetes Care 22:789–794
Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M (2003) Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP-NIDDM Trial. JAMA 290:486–494
Home PD, Pocock SJ, Beck-Nielsen H, RECORD Study Group et al (2007) Rosiglitazone evaluated for cardiovascular outcomes—an interim analysis. N Engl J Med 357:28–38
Singh S, Loke YK, Furberg CD (2007) Thiazolidinediones and heart failure: a teleoanalysis. Diabetes Care 30:2248–2254
Khan MA, St Peter JV, Xue JL (2002) A prospective, randomized comparison of the metabolic effects of pioglitazone or rosiglitazone in patients with type 2 diabetes who were previously treated with troglitazone. Diabetes Care 25:708–711
Goldberg RB, Kendall DM, Deeg MA, GLAI Study Investigators et al (2005) A comparison of lipid and glycemic effects of pioglitazone and rosiglitazone in patients with type 2 diabetes and dyslipidemia. Diabetes Care 28:1547–1554
Nissen SE, Wolski K (2007) Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 356:2457–2471
Singh S, Loke YK, Furberg CD (2007) Long-term risk of cardiovascular events with rosiglitazone: a meta-analysis. JAMA 298:1189–1195
Dormandy JA, Charbonnel B, Eckland DJA et al (2005) Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive (PROspective pioglitAzone Clinical Trial in macrovascular Events): a randomized controlled trial. Lancet 366:1279–1289
Lincoff AM, Wolski K, Nicholls SJ, Nissen SE (2007) Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of randomized trials. JAMA 298:1180–1188
Nathan DM, Buse JB, Davidson MB et al (2008) Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy. Update regarding the thiazolidinediones. Diabetologia 51:8–11
Meier C, Kraenzlin ME, Bodmer M, Jick SS, Jick H, Meier CR (2008) Use of thiazolidinediones and fracture risk. Arch Intern Med 168:820–825
Horvath K, Jeitler K, Berghold A et al (2007) Long-acting insulin analogues versus NPH insulin (human isophane insulin) for type 2 diabetes mellitus. Cochrane Database Syst Rev 2:CD005613
Raskin P, Allen E, Hollander P (2005) Initiating insulin therapy in type 2 diabetes. Diabetes Care 28:260–265
Dailey G, Rosenstock J, Moses RG, Ways K (2004) Insulin glulisine provides improved glycemic control in patients with type 2 diabetes. Diabetes Care 27:2363–2368 80
Nathan DM, Roussell A, Godine JE (1988) Glyburide or insulin for metabolic control in non-insulin-dependent diabetes mellitus: a randomized double-blind study. Ann Int Med 108:334–340
Abraira C, Johnson N, Colwell J, VA CSDM Group (1995) VA Cooperative study on glycemic control and complications in type II diabetes. Diabetes Care 18:1113–1123
Zammitt NN, Frier BM (2005) Hypoglycemia in type 2 diabetes. Diabetes Care 28:2948–2961
Miller CD, Phillips LS, Ziemer DC, Gallina DL, Cook CB, El-Kebbi IM (2005) Hypoglycemia in patients with type 2 diabetes mellitus. Arch Int Med 161:1653–1659
Kendall DM, Riddle MC, Rosenstock J et al (2005) Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care 28:1083–1091
DeFronzo RA, Ratner RE, Han J et al (2005) Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care 28:1092–1100
Buse JB, Henry RR, Han J, Kim DD, Fineman MS, Baron AD (2005) Exenatide-113 Clinical Study Group. Effects of exenatide on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care 27:2628–35
Heine RJ, Van Gaal LF, Johns D, Mihm MJ, Widel MH, Brodows RG (2005) Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes. Ann Int Med 143:559–569
Riddle M, Frias J, Zhang B et al (2007) Pramlintide improved glycemic control and reduced weight in patients with type 2 diabetes using basal insulin. Diabetes Care 30:2794–2799
Raz I, Hanefeld M, Xu L, Caria C, Davies M, Williams-Herman D (2006) Sitagliptin Study 023 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy in patients with type 2 diabetes mellitus. Diabetologia 49:2564–2571
Goldstein B, Feinglos M, Lunceford J, Johnson J, Williams-Herman D (2007) Sitagliptin 036 Study Group. Effect of initial combination therapy with sitagliptin, a dipeptidyl peptidase-4 inhibitor, and metformin on glycemic control in patients with type 2 diabetes. Diabetes Care 30:1979–1987
Welschen LMC, Bloemendal E, Nijpels G et al (2005) Self-monitoring of blood glucose in patients with type 2 diabetes who are not using insulin: a systematic review. Diabetes Care 28:1510–1517
Farmer A, Wade A, Goyder E et al (2007) Impact of self monitoring of blood glucose in the management of patients with non-insulin treated diabetes: open parallel group randomised trial. BMJ 335:132
Ilkova H, Glaser B, Tunckale A, Bagriacik N, Cerasi E (1997) Induction of long-term glycemic control in newly diagnosed type 2 diabetic patients by transient intensive insulin treatment. Diabetes Care 20:1353–1356
Weng J, Li Y, Xu W et al (2008) Effect of intensive insulin therapy on beta-cell function and glycemic control in patients with newly diagnosed type 2 diabetes: a multicentre randomized parallel-group trial. Lancet 371:1753–1760
UKPDS Group (1995) UK Prospective Diabetes Study 16: overview of six years’ therapy of type 2 diabetes: a progressive disease. Diabetes 44:1249–1258
Hirsch IB, Bergenstal RM, Parkin CG, Wright E, Buse JB (2005) A real-world approach to insulin therapy in primary care practice. Clinical Diabetes 23:78–86
Schwartz S, Sievers R, Strange P, Lyness WH, Hollander P (2003) Insulin 70/30 mix plus metformin versus triple oral therapy in the treatment of type 2 diabetes after failure of two oral drugs. Diabetes Care 26:2238–2243
Yki-Jarvinen H, Ryysy L, Nikkila K, Tulokas T, Vanamo R, Heikkila M (1999) Comparison of bedtime insulin regimens in patients with type 2 diabetes mellitus. Ann Int Med 130:389–396
Author information
Authors and Affiliations
Corresponding author
Additional information
Simultaneous publication: This article is being simultaneously published in 2008 in Diabetes Care and Diabetologia by the American Diabetes Association and the European Association for the Study of Diabetes. Copyright 2008 by the American Diabetes Association and Springer. Copying with attribution allowed for any non-commercial use of the work.
Rights and permissions
About this article
Cite this article
Nathan, D.M., Buse, J.B., Davidson, M.B. et al. Medical management of hyperglycaemia in type 2 diabetes mellitus: a consensus algorithm for the initiation and adjustment of therapy. Diabetologia 52, 17–30 (2009). https://doi.org/10.1007/s00125-008-1157-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-008-1157-y