Abstract
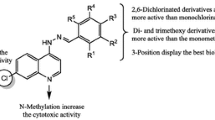
Heteroaromatic derivatives (3a–f) have been synthesized and evaluated for their activity against four cancer cell lines. Among the studied compounds, 1-(7-Chloroquinolin-4-yl)-2-[(1H-pyrrol-2-yl)methylene]hydrazine (3e) exhibited an excellent cytotoxic activity against the referred lines, and especially on melanoma cells (MDAMB-435). In this case, compound 3e is four times more active than the standard substance Doxorubicin. Together with other results from our group, 7-chloro-4-quinolinylhydrazones derived from chloroquine could be considered a relevant finding toward the rational design of new leads for antitumor compounds.


Similar content being viewed by others
References
Ahmed SA, Gogal RM Jr, Walsh JE (1994) A new rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: An alternative to [3H]thymidine incorporation assay. J Immunol Methods 170:211–224
Augustijns P, Geusens P, Verbeke N (1992) Chloroquine levels in blood during chronic treatment of patients with rheumatoid arthritis. Eur J Clin Pharmacol 42:429–433
Chuandong F, Wang W, Zhao B, Zhang S, Miao J (2006) Chloroquine inhibits cell growth and induces cell death in A549 lung cancer cells. Bioorg Med Chem 14:3218–3222. doi:10.1016/j.bmc.2005.12.035
Fattorusso C, Campiani G, Kukreja G, Persico M, Butini S, Romano MP, Altarelli M, Ros S, Brindisi M, Savini L, Novellino E, Nacci V, Fattorusso E, Parapini S, Basilico N, Taramelli D, Yardley V, Croft S, Borriello M, Gemma S (2008) Design, synthesis, and structure-activity relationship studies of 4-quinolinyl- and 9-acrydinylhydrazones as potent antimalarial agents. J Med Chem 51:1333–1343. doi:10.1021/jm7012375
Ferreira ML, Gonçalves RSB, Cardoso LNF, Kaiser CR, Candéa ALP, Henriques MGO, Lourenço MCS, Bezerra FAFM, Souza MVN (2010) Synthesis and antitubercular activity of heteroaromatic isonicotinoyl and 7-chloro-4-quinolinyl hydrazone derivatives. TheScientificWorldJOURNAL 10:1347–1355. doi:10.1100/tsw.2010.124
Foley M, Tilley L (1998) Quinoline antimalarials: mechanisms of action and resistance and prospects for new agents. Pharmacol Ther 79:55–87. doi:10.1016/S0163-7258(98)00012-6
Font M, Monge A, Ruiz I, Heras B (1997) Structure-activity relationships in quinoline Reissert derivatives with HIV-1 reverse transcriptase inhibitory activity. Drug Des Discov 14:259–272
Kaminsky D, Meltzer RI (1968) Quinolone antibacterial agents: Oxolinic acid and related compounds. J Med Chem 11:160–163. doi:10.1021/jm00307a041
Kim EL, Wüstenberg R, Rübsam A, Schmitz-Salue C, Warnecke G, Bücker EM, Pettkus N, Speidel D, Rohde V, Schulz-Schaeffer W, Deppert W, Giese A (2010) Chloroquine activates the p53 pathway and induces apoptosis in human glioma cells. Neuro Oncol 12:389–400
Krause W, Jordan A, Scholz R, Jimenez JLM (2005) Iodinated nitroimidazoles as radiosensitizers. Anticancer Res 25:2145–2151
Montenegro RC, Lotufo LV, Moraes MO, Pessoa CO, Rodrigues FAR, Bispo MLF, Cardoso LNF, Kaiser CR, Souza MVN (2011) Synthesis and antitumoral evaluation of 7-chloro-4-quinolinylhydrazones derivatives. Med Chem 7:599–604
Musiol R, Jampilek J, Buchta V, Silva L, Niedbala H, Podeszwa B, Palka A, Majerz-Maniecka K, Oleksyn B, Polanski J (2006) Antifungal properties of new series of quinoline derivatives. Bioorg Med Chem 14:3592–3598. doi:10.1016/j.bmc.2006.01.016
Savarino A, Di Trani L, Donatelli I, Cauda R, Cassone A (2006) New insights into the antiviral effects of chloroquine. Lancet Infect Dis 6:67–69
Sharma P, Sharma JD (2001) In vitro hemolysis of human erythrocytes—by plant extracts with antiplasmodial activity. J Ethnopharmacol 74:239–243
Sloboda AE, Powell D, Poletto JF, Pickett WC, Gibbons JJJr, Bell DH, Oronsky AL, Kerwar SS (1991) Antiinflammatory and antiarthritic properties of a substituted quinoline carboxylic acid: CL 306, 293. J Rheumatol 18:855–860
Warshakoon NC, Sheville J, Bhatt RT, Ji W, Mendez-Andino JL, Meyers KM, Kim N, Wos JA, Mitchell C, Paris JL, Pinney BB, Reizes O, Hu XE (2006) Design and synthesis of substituted quinolines as novel and selective melanin concentrating hormone antagonists as anti-obesity agents. Bioorg Med Chem Lett 16:5207–5211
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Montenegro, R.C., Lotufo, L.V., de Moraes, M.O. et al. 1-(7-Chloroquinolin-4-yl)-2-[(1H-pyrrol-2-yl)methylene]hydrazine: a potent compound against cancer. Med Chem Res 21, 3615–3619 (2012). https://doi.org/10.1007/s00044-011-9894-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-011-9894-8